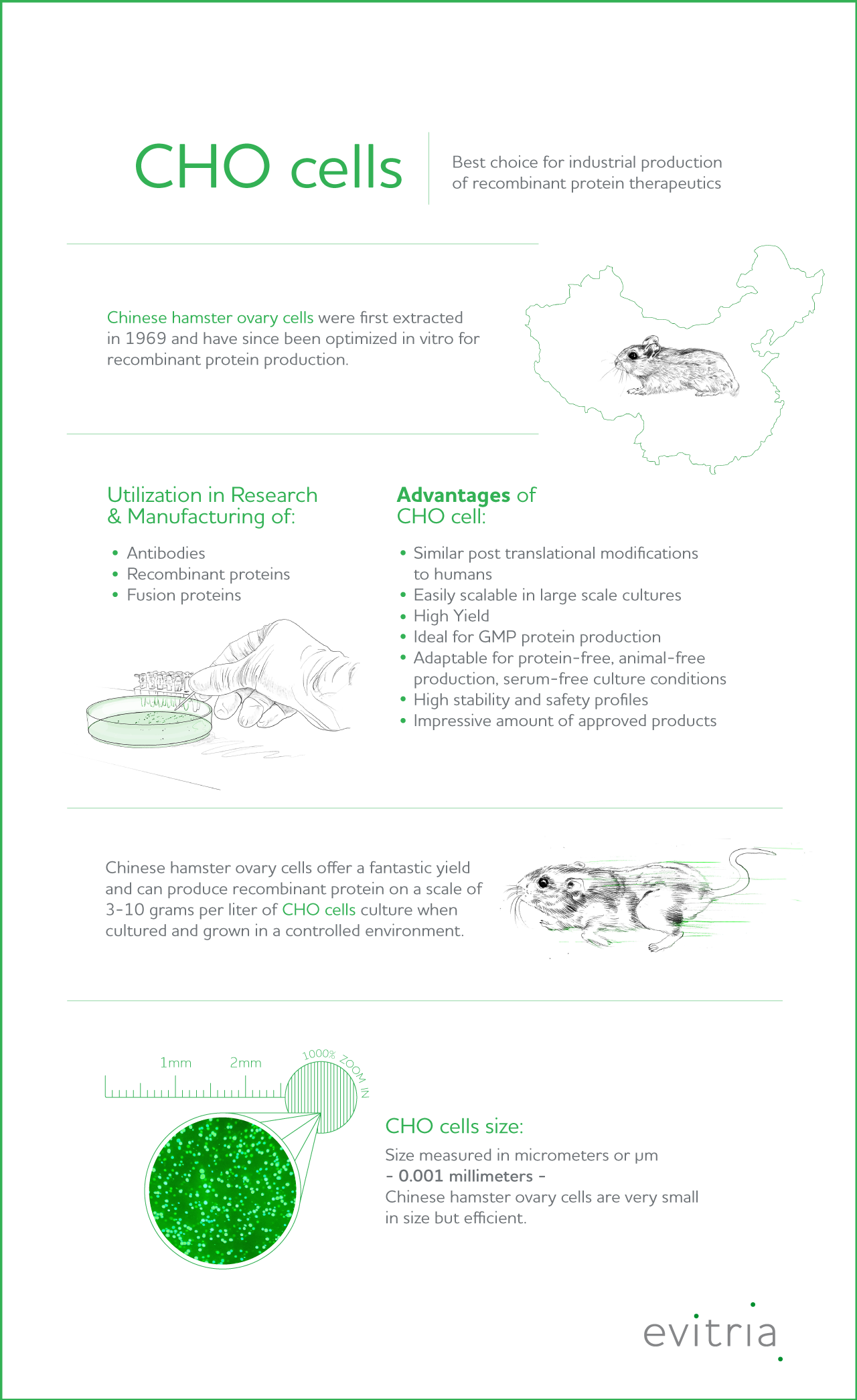
CHO cells (Chinese hamster ovary cells) have been cultured and manipulated for the use in molecular biology and pharmaceutical biotechnology for decades. While many different cell lines are used in molecular biology research labs, CHO cells are the most commonly used host for the expression of recombinant antibodies.
In fact, CHO cell lines are the best choice as a mammalian host for industrial production of recombinant protein therapeutics. Why? They are highly efficient in terms of yield. But more about the potential of this mammalian host in gene expression later on.
In this article, we want to cover the following questions:
As the name suggests, Chinese hamster ovary cells or in short CHO cells are derived from the ovary of a small rodent called the Chinese hamster.
Subscribe to our Newsletter
Get all the latest updates, and learn about our advancements in antibody production.
Subscribe now
Due to their similarity to the human cell system, CHO cells are used in biological and medical research, primarily such as genomic and chromosome studies, toxicity assays and gene expression.
One of the CHO cells’ main uses is to express recombinant proteins in bioreactors. They can produce recombinant protein on a scale of 3-10 grams per liter of CHO cells culture.1
The protein synthesis machinery within a CHO cell produces proteins that are characterized by similar post-translation modifications as human cells, and so these proteins are suitable for human applications, which is why they are used in the production of therapeutic proteins.
Chinese hamster ovary cells remain a popular choice in the efficient production of:
The choice of the right host for the biological synthesis of chemical compounds or proteins is a crucial factor in the production of pharmaceuticals.
This is why the cells of Chinese hamsters are the first choice in pharmaceutical research and development. In the history of antibodies CHO cells have been used for lab purposes from as early as 1919.
The original CHO cells for the cell line CHO-K1 were isolated by Theodore Puck from an ovary once in 1968, and after cell line development and cell engineering, they have been multiplied by adherent and suspension culture ever since – without harming a single animal during the entire production process for CHO cells biopharmaceuticals.
Chinese hamster ovary cells as mammalian cell line expression systems offer several advantages, the most important being their ability to produce proteins with complex glycosylations, post-translational modifications (PTMs), that are similar to those produced in humans. These modifications within recombinant protein production are not possible in other popular in vitro cell types such as E. coli.
As an added benefit, CHO cell growth and viability is easy to achieve in large-scale bioprocesses under defined conditions, rendering them ideal for GMP protein production procedures.
With their tolerance to variations in parameters such as pH, oxygen levels, temperature or cell density, they are the ideal host cell for large-scale culture while at the same time offering high yields of recombinant protein expression and specific productivity.
Thanks to these characteristics as well as constant progress, optimization and improvement, CHO cells cultures today can be used for large-scale transient expression after plasmid transfection in a relatively short timespan. CHO cell antibody production can be tailored to the respective customer’s requirements.
All advantages at a glance:
Where do CHO cells come from, you may ask. Well, one would think that the name gives it away: Chinese hamster ovary cells are derived from the mammal with the same name, the Chinese hamster, or cricetulus griseus.
Researchers have found their cell structure to be similar to that of the human body, which is why their cells are regarded as an ideal tool in the development and production of pharmaceuticals.
To that end they are generated and modified in labs – and here is another benefit and little-known fact: They have a fantastic yield, adding to their allure.
True to their reputation of being real powerhouses, Chinese hamster ovary cells offer a fantastic yield and can produce recombinant protein on a scale of 3-10 grams per liter of CHO cell culture when cultured and grown in a controlled environment.
However, besides reproductivity they also score with some other pretty cool qualities, one of which is their longevity.
One of the key requirements of CHO cells is that they must be able to survive and proliferate in vitro – in other words, outside of the body and in a lab environment.
CHO cells are considered immortal: They can be extensively multiplied, which, however, does come with drawbacks. This is because, despite being immortal, it is in the character of CHO cells to have a high propensity to pick up genetic mutations, and so the same culture cannot be continued forever.
In case you wonder why we use CHO cells, then the answer – for once – is rather simple. They are one of the primary cells utilized in the research and manufacturing of
As already mentioned, they offer a fantastic yield, which is obviously key in any market-driven industry. But surely this cannot be enough to make them such a popular choice?
No, offering a fantastic yield is not enough in today’s tough and contested markets; neither is being versatile and resilient. Much rather, it is their similarity to the human cell system that makes CHO cells such a popular tool for genetic studies, toxicity screening and gene expression.
As such, they form the vital basis for a vast number of applications and are perfect tools for research and development.
One of the most astonishing facts about CHO cells is their size – or actually the lack thereof. You may wonder: How big is a CHO cell?
With a size measured in micrometers or μm – yes, that’s 0.001 millimeters – Chinese hamster ovary cells are infinitesimal.
To start out with we should define what “adherent” actually means: Adherent cells have to be attached to a surface in order to grow, while suspension cells grow in solution.
Such cell types can be subcultured by simply taking a small volume of the parent culture and diluting it in a fresh growth medium. Over time, the medium can be gradually changed, and the cells slowly adapt to their new surroundings. CHO cells are naturally adherent but can be adapted over time to grow in suspension.
As a leading service provider for recombinant antibody expression in CHO cells, evitria combines Swiss thoroughness with an innovative spirit.
In line with the requirements of today’s market, the objective is to offer both a fast project execution and highest quality standards, in other words: Maximum quality delivered in a minimum of time:
Fast project execution …
… applies to all phases from project inception to shipment of the final product:
Highest quality standards …
… are adhered to and delivered throughout all processes of every project. evitria focuses on one service only, namely the transient antibody expression in CHO cells. This allows customers to expect guaranteed delivery with respect to:
From the Swiss headquarters, evitria delivers worldwide and provides high quality CHO cells for the use in an ever-growing portfolio of biotech drugs.
The production of high quality, reproducible material is critical for the development of antibody-based therapeutics. The evitria-Genovis workflow combines rapid, high quality, antibody production with high-throughput mass spectrometry for greater insight and control of key quality attributes.
Download the poster to learn more.

CHO cells is the abbreviation for Chinese hamster ovary cells. As an epithelial cell line with several advantages for biopharma, they are commonly used in the production of recombinant proteins as well as in scientific research.
A cell culture medium imitates an environment in a growth medium in the laboratory to ensure the healthy growth of cells outside their natural tissue. It is a source of energy and contains compounds which regulate the cell cycle like a complement of amino acids, vitamins, inorganic salts, glucose, and serum as a source of growth factors, hormones, and attachment factors.
CHO cells are frequently used by researchers in medicine and biology. Moreover, they are a key element in the commercialized production of therapeutics based on recombinant proteins. At evitria we use CHO cells for custom recombinant antibody production.
Chinese hamster ovary cells derive from mammals. In 1968, cells were extracted from a Chinese hamster’s ovaries and have since then been multiplied and optimized in vitro. By making them viable outside the rodent’s body, they can be reproduced indefinitely.
A plasmid vector containing the genetic sequence of an antibody can be inserted into the CHO cell, causing the CHO cell to produce the antibody. If the vector remains within the cytoplasm of the cell, the expression is known as “transient expression”. If the vector integrates within the CHO cell genome, a “stable” expression process occurs. This mechanism is a defining key stone in the production of antibody pharmaceuticals.
Chinese hamster ovary cells are a very commonly relied-on component in the antibody production process, given their ability to produce even complex proteins similar to those in humans. Their high tolerance for variations in pH, oxygen levels, temperature or cell density makes them ideal for large-scale manufacturing. This adds to them being extremely stable and adaptable for protein-free, animal-free production and serum-free culture conditions.
The Chinese hamster (cricetulus griseus) is a rodent native to the deserts of northern China and Mongolia.
Unlike other hamsters with their stubby tails, the Chinese hamster flaunts an uncommonly long tail of approximately 20 to 30 mm (0.8 to 1.3 inches) and is generally longer. It is a solitary animal, with a life expectancy of approximately two to three years.
Researchers have found the cell structure of the Chinese hamster to be similar to that of the human body. As such, they have proven to be an ideal tool in the development and production of pharmaceuticals.
It hardly comes as a surprise then that with regards to the industrial-scale production of recombinant protein compounds, CHO cells are the most commonly used cells derived from mammalian hosts.