Co-written by Aarti Narang, former Associate Scientific Director at evitria.
Proteins tagged with 4-10 histidine (His) residues can be purified using Immobilized Metal Affinity Chromatography (IMAC). This technique utilizes imidazole rings present on histidine side chains to non-covalently bind to the positively charged metal ions within an IMAC resin. IMAC purification can be used to purify proteins in their native conformation as well as under denaturing conditions. This versatility has made the addition of a His-tag a common choice to simplify the protein purification process.
During purification, the sample is loaded at a pH above 6, which causes the imidazole rings of the histidines to become deprotonated and allows them to bind to positively charged metal ions, such as Nickel or Cobalt. After washing to remove non-tagged proteins, the target protein can be eluted either by lowering the pH to protonate the histidine residues or by adding high concentrations of free imidazole to the elution buffer. The high concentration imidazole in the elution buffer outcompetes the His-tag for binding and effectively elutes the target protein while maintaining a neutral pH. This elution strategy is very mild to the target protein, but often the imidazole in the eluate must be removed using a desalting chromatography column or dialysis.
The His-tag can be placed at either the N- or C-terminus of the protein and optimal placement is protein-specific. Linker sequences (e.g. Glycine-Serine repeats) may be used between the His-tag and the target protein in order to reduce steric hinderance of the histidine binding reaction. A protease cleavage site can also be included as part of this linker to allow for removal of the His-tag. Commonly, a tobacco etch virus (TEV) protease site is included immediately after an N-terminal His-tag. This leaves only an N-terminal Glycine or Serine on the target protein after digestion with TEV-protease.
Subscribe to our Newsletter
Get all the latest updates, and learn about our advancements in antibody production.
Subscribe now
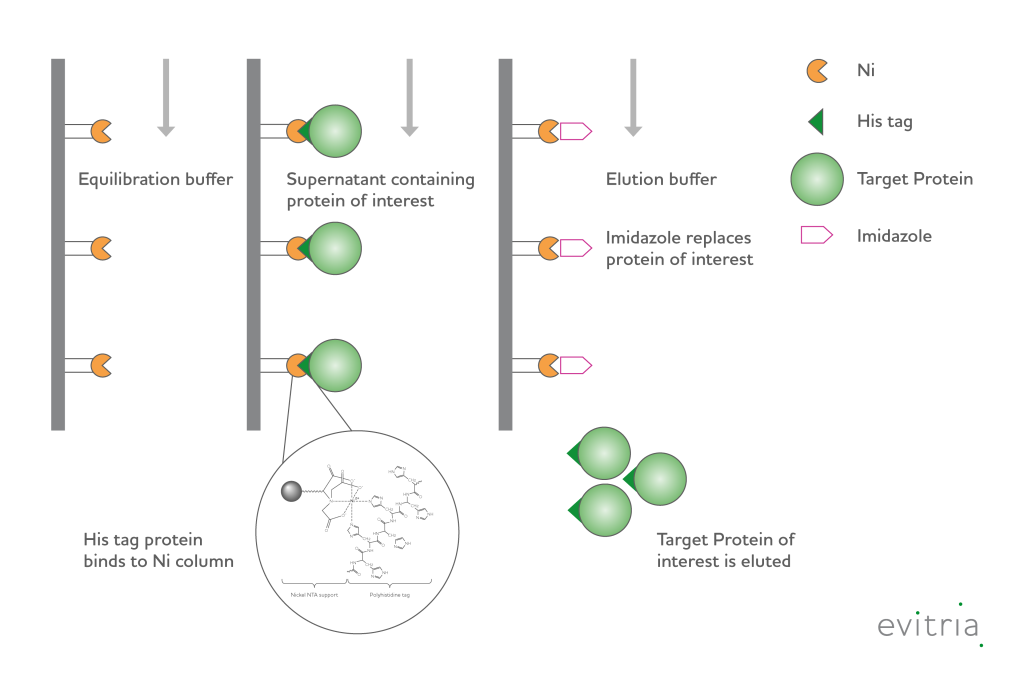
Purification of His-tagged proteins from CHO-cell supernatants require IMAC columns especially developed for this purpose, since the large volume and presence of chelating agents can result in significant metal ion leaching. If CHO-specific IMAC columns are not used, it may cause decreased protein binding capacity and result in loss of target protein recovery.
His-tagged proteins purified by evitria AG are eluted using imidazole then directly loaded onto a desalting column for buffer exchange into phosphate buffered saline. If needed, a subsequent polishing step using gel filtration can be performed to remove residual contaminants and provide a high quality, high purity preparation.