evitria AG offers recombinant antibodies for industrial applications, small-scale usage or discovery research.
Our main focus is with in vitro CHO cell culture-based recombinant antibody production and expression of antibody-related proteins such as Fabs and multi-specific antibodies and even tagged antigens.
Transient Antibody expression in CHO cells is much faster than in comparable cell lines. Our expression platform allows custom antibody production in large scales with very low levels of endotoxins (<1EU/mg) in 5 weeks.
Our recombinant antibody production service includes custom development and manufacturing of the following products:
Antibody production runs
Antibodies purified
Projects completed on time
From Sequence to Antibody
Recombinant antibody production is our focus in which we are constantly developing. Since 2010, we have preformed more than 120,000 antibody production runs, expressed more than 23,000 proteins and purified more than 20,000 antibodies.
We have been supporting customers globally from the Americas, Europe, Asia and Australia ranging from academic laboratories, small biotech start-ups to global biopharmaceutical companies.
Whether you are looking to entrust a partner with your complete antibody production or to manage capacity bottlenecks with single projects, you can benefit from our expertise.
This unique expertise includes the following capabilities:
From sequence to antibody in 5 weeks
Project start
0-1 business days
Pilot study
(from electronic sequence to quantified pilot supernatant)
15-20 business days
Larger-scale expression
5-10 business days
Purification
2-3 business days

Final analytics
2-3 business days
Shipment
1-2 business days
Antibody production with us allows flexible scalability to supply companies in discovery research and commercial manufacturing. We strive to offer you the best possible custom recombinant antibody in the highest quality.
Therefore, we use CHO cells antibody production to express our recombinant monoclonal antibodies, easily surpassing functionality and reliability of competing polyclonal antibodies and traditional hybridoma-derived monoclonal antibody products.
We are able to offer large-scale recombinant antibody production services to express antibodies with low levels of endotoxin (<1 EU/mg), either with or without afucosylation. Moreover, CHO cell antibody production is much faster than any traditional technique. We are up to months faster than antibody suppliers relying on hybridoma cells.
We offer afucosylated (ADCC enhanced) antibody production. ADCC (antibody-dependent cytotoxicity) is a mechanism of the immune system to fight infected and tumorigenic cells. Therapeutic antibodies with an artificially lowered fucose-content show enhanced ADCC activity and therefore increased potency and reduction in side-effects.
Since CHO cells are stable cell lines originating from mammals, their antibodies are posttranslationally modified with oligosaccarides. Many modern Ab applications call for intact glycosylation patterns and our mammalian cell lines take care of this requirement.
Antibodies lacking fucose are termed afucosylated antibodies and were found to be promising drug candidates and research tools due to enhancement of ADCC. The impact of those glycosylation modifications is the subject of numerous research projects in pharmaceutical companies and academic institutions. By virtue of our exclusive licensing partnership, we offer afucosylation via ProBioGen’s GlymaxX® technology to our customers.
More about Afucosylation Service
Antibodies with reduced Fc-effector functions are essential for therapeutic applications where activation of the host’s immune system would have negative effects, such as when an antibody is used to neutralize a binding interaction.
We hold a licensing partnership to offer antibodies with truly silent Fc domains, using mAbsolve’s STR technology. STR-modified antibodies show no binding or activity above background noise across the Fc gamma receptors, far surpassing all previously described modifications.
More about Fc-silencing Service
Your protein production needs to go beyond recombinant antibody expression? The recombinant antibody expression platform at evitria is flexible. We can meet your needs for any secreted proteins (scFvs, soluble extracellular domains, cytokines, viral proteins and more). Other examples for proteins are various research reagents for e.g. ELISA and fluorescence assays, inhibitors, receptors for small molecules, protein folding and many more.
We deliver nano-filtered, sterile supernatant for your established inhouse downstream processes. Get in touch with us to obtain more information.
More about other protein expression
Stay up-to-date with the latest news directly in your inbox.
Subscribe to our Newsletter
Get all the latest updates, and learn about our advancements in antibody production.
Subscribe now
The production of high quality, reproducible material is critical for the development of antibody-based therapeutics. The evitria-Genovis workflow combines rapid, high quality, antibody production with high-throughput mass spectrometry for greater insight and control of key quality attributes.
Download the poster to learn more.

We at evitria have a track record of over 120.000 transfections and purified more than 20.000 antibodies for international customers.
We have accumulated a wealth of experience and offer our expertise to serve our customers as a contract research organization, in short CRO, for recombinant antibody expression.
Our transient recombinant antibody expression platform leverages the advantages of CHO cells. Our processes are independent of laboratory animals and their immunization, therefore our recombinant antibodies are free of animal contaminations.
Our CHO based expression platform enables us to guarantee shorter timelines and higher yields than possible with traditional, out-dated methods.
Overview:
We are up to months faster than competitors. We commence projects within one day of customer’s approval and complete a pilot study within 15 – 20 days. The subsequent on-scale expression takes 5 – 10 days and the product isolation is typically finished after 2 – 3 days.
Next, the final analytics are completed within 2 – 3 days. To conclude, we hand over the deliverables to our logistics partner and have them delivered to you in 1 – 2 business days. Our typical project timeline from start to finish is five weeks.
Overview:
No, nor do we use E.Coli or HEK cells. Instead, we use mammalian cell cultures. We prefer Chinese Hamster Ovarian (CHO) cells in our mammalian expression system, since that comes with numerous advantages over the use of laboratory animals.
Recombinant antibody expression in CHO allows large-scale recombinant antibody production with low levels of endotoxin (<1 EU/mg). Moreover, CHO cell antibody production is much faster than any traditional technique. We are up to months faster than antibody suppliers relying on hybridoma cells.
Mammalian expression is the process of biological protein production in mammals or mammalian cells, such as HEK293 or CHO cells. Some proteins, such as antibodies, can’t be produced in fully functional form in other cell cultures.
evitria offers custom antibodies to their customers. Our service includes recombinant antibodies, afucosylated antibodies or other proteins. In contrast to a conventional antibody supplier, we do not offer a catalog with standardized antibodies.
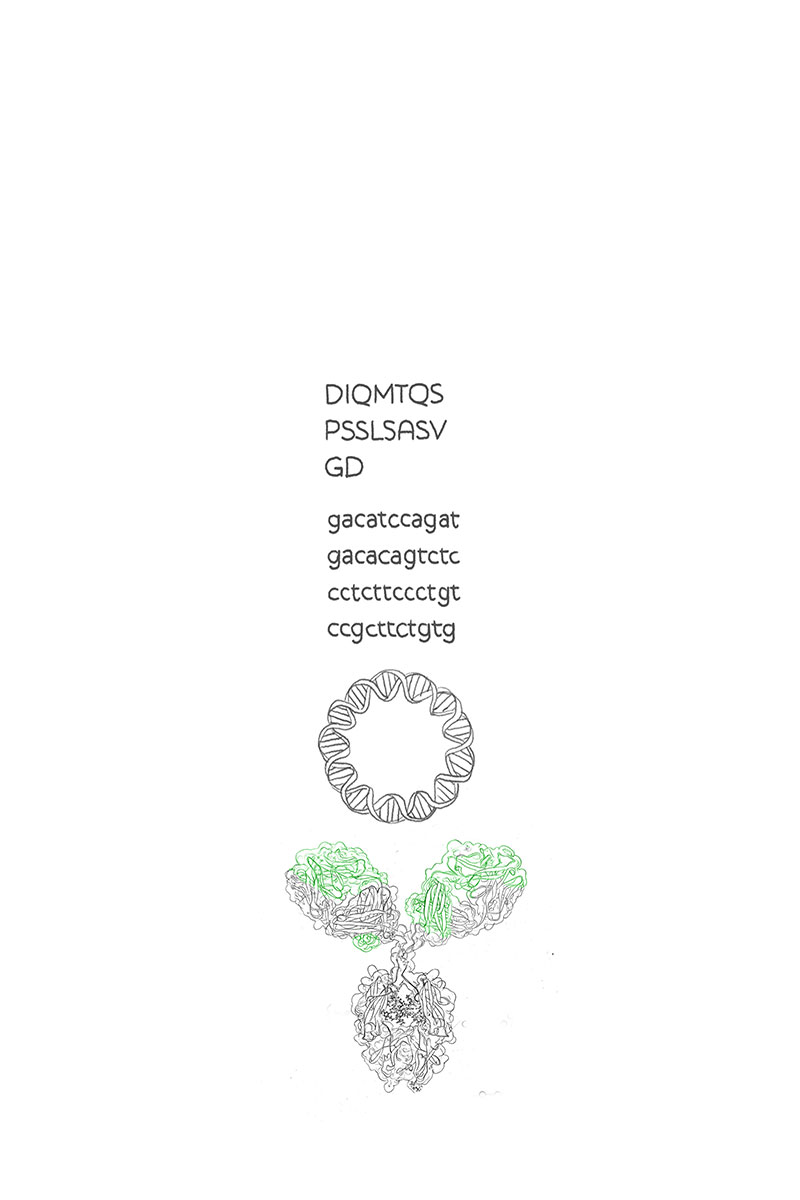
Antibody engineering is the process of changing and optimizing antibodies to equip them with better or novel properties. In this case, the fab fragment is modified. One example is the fine-tuning of antigen binding sites to enhance affinity or specificity towards an antigen.
Another example is the fusion of two antibodies with distinct antigens, to form a bispecific antibody that shows affinity towards both antigens.
Protein A purification is standard and we use alternative resins such as Protein G, L, Kappa/Lambda-Select or anti-IgG-CH1 as needed. An additional size exclusion chromatography (SEC) purification step can be added, as well as ion exchange chromatography. Please inquire for more details.
Read more on recombinant antibody purification
Our average timeline is between 6 and 7 weeks for new antibodies. We do guarantee 6-8 weeks from amino acid sequence to purified antibody with our standard services and 5-6 weeks with our RUSH offering. If we have produced the antibody before then reproduction will take 1-2 weeks.
We have produced both bispecific and trispecific antibodies in various formats. Please contact your local evitria representative to discuss your specific project.
No, but please see our “Partners” page to find best-in-class solutions for required services.
No, but please see our “Partners” page to find best-in-class solutions for required services. We work together with established partners for optimization of antibody development workflows.
We are unable to send our proprietary expression vectors but can clone into a freedom-to-use vector and send for an additional fee.
There are various different antibody formats, e.g. full-length, VHH, bispecific or chimeric abs.
A recombinant gene is a sequence of genetic code that is inserted into different genetic material by biotechnological techniques. Recombinant gene sequences (previously undergone the process of gene synthesis) are used to transfer blueprints of proteins (e. g. recombinant antibodies) into cells in order to have them produce said protein.
No, not so far. Please contact your local evitria salesperson to discuss your individual project.
At evitria, we focus on transient antibody production. Thereby, we rely on CHO cells as our expression system of choice, as they come with several advantages in terms of quality and speed.
No, evitria works with partners to ensure maximum value to customers. For sequencing issues Rapid Novor is evitria’s partner.
Do you still have questions? Read all the answers to frequently asked questions on the FAQ page or send us a noncommittal request.