Custom recombinant antibody production
We are a leading service provider for custom recombinant antibody production. Get in contact!
There are different companies specializing in custom antibody production as a service to support pharmaceutical companies and research organizations.
In many cases, it can be beneficial to outsource different steps or the entire process of polyclonal, monoclonal or recombinant antibody expression, as it requires a lot of expertise and modern equipment. Especially custom recombinant antibody production services have become an important part of medical research and the development of biopharmaceuticals.1
In the race for the development of new antibody drugs, many antibody production companies and research organizations rely on contract research organizations (CROs) and contract development and manufacturing organizations (CDMOs).
By outsourcing different steps of the antibody development process, it is often possible to improve time and co-management. As the production of monoclonal antibodies and polyclonal antibodies requires a lot of expertise and the right equipment, many companies turn to CROs and CDMOs for technical support.
Subscribe to our Newsletter
Get all the latest updates, and learn about our advancements in antibody production.
Subscribe now
Custom antibody production is a quickly changing sector that requires equipment which is compatible to the newest standards in order to be economically competitive. Further, antibody production has to comply with current good manufacturing practices (cGMPs) as determined by the World Health Organization (WHO).
Outsourcing can help companies to concentrate on their core business and is often more cost lucrative than opening a new department or buying new equipment. Additionally, one can profit from the specialization of CROs and CDMOs, often focusing on specific steps in antibody production. Nevertheless, there are many different service providers on the market, and with ever-changing technologies and regulatory standards as well as logistic considerations, it can be difficult to determine which CRO or CDMO is best suited for one’s project.
There is an astonishing number of antibody production services available on the market. These include the development process of antibodies and its different steps, such as antigen design, the immunization of a host, antibody generation in hybridoma cell lines, antibody purification and regular assays to ensure the absence of impurities.
Furthermore, antibodies are widely used in drug conjugations. Consequently. there is a number of CROs that offer the production of antibody drug conjugates, as well. Depending on the desired type of antibody, the individual manufacturing steps can look differently.
If you are thinking about outsourcing antibody production or different steps of antibody production, there are different matters to consider. At first, the type of antibody determines the production process. Not every CRO or CDMO offers every kind of production, as they require different equipment.
Further, the CRO has to match your expectations in regard to costs and time efficiency and needs to meet the country-specific regulatory requirements.
There are various antibody types with different kinds of appliances. We distinguish between polyclonal antibodies, monoclonal antibodies, and recombinant antibodies. Different custom antibody services are available for different purposes.
While some custom antibody production services offer the outsourcing of a whole antibody project, a lot of organizations specialize in one or more production steps. As they are experts in their field of custom antibody development and have high-quality equipment at hand, it becomes possible to produce antibodies faster and on a large scale.
However, this can only be achieved if the CRO or CDMO lives up to the expectations and demands of the client. It is therefore very important to choose a trustworthy partner for the development of a specific antibody.
As we have already established, the development of new antibodies has to follow different provisions to guarantee a safe product. It is therefore vital to choose a CRO with experience in country specific GMP regulations.
GMP standards have gained in significance since the SARS-Cov-2 pandemic and the development of the Covid-19 vaccination reagents which relied on efficient production with reliable quality controls of starting materials, as well as product validations and characterization through ELISA kits or Western blotting in custom monoclonal antibody services, which were often less than satisfactory in several countries.2 Without the right technologies, it can be difficult to meet standards when handling sensitive formulations and substances like antibody drugs.
Not every antibody production project runs on a larger scale. Sometimes, recombinant protein or monoclonal antibody production of smaller batches are needed for immunology research purposes. It is therefore vital to investigate if the CRO of interest is able to live up to your standards and offers scalable production services.
The production of different antibody types relies on different expression systems. While in vivo antibodies are produced in living animals, recombinant antibody production has the advantage of in vitro antibody generation by using mammalian host cell lines.
Two of the most established cell lines are human embryonic kidney cells (HEK cells) and Chinese hamster ovary cells (CHO cells). As CHO cells grow well in dispense culture and are less prone to immunogenicity, they are evitria’s cell line of choice.
Further, e.Coli expression systems can be used to generate less complex structures. However, mammalian expression systems are more suitable for the generation of full-length proteins.
We are a leading service provider for custom recombinant antibody production. Get in contact!
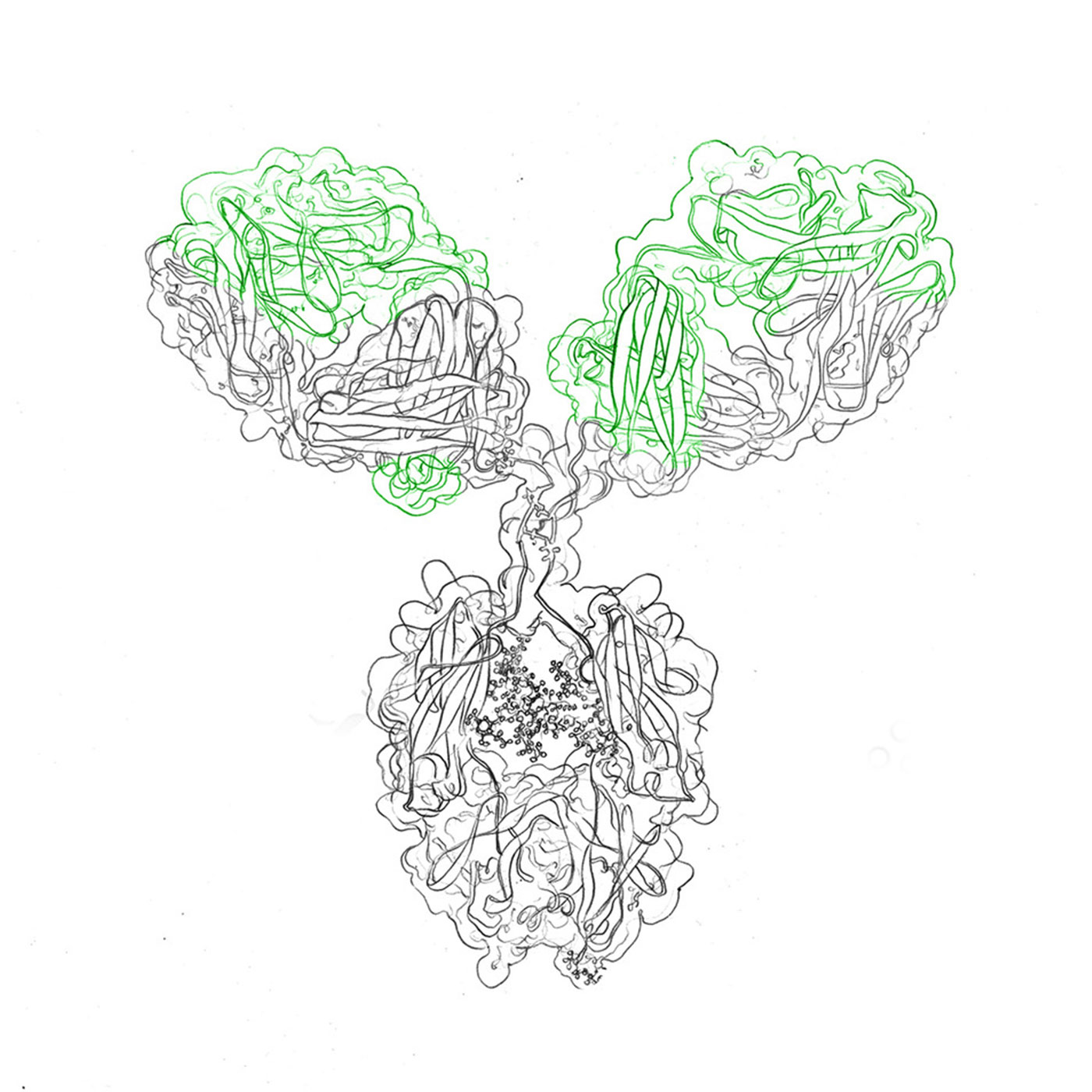
With new advances in technology and research, custom recombinant antibody expression services have become an important segment of the pharmaceutical sector. evitria offers a variety of services from the production of recombinant antibodies, afucosylated antibodies and other proteins.
As CHO cells have proven themselves as valuable expression systems for full-length proteins due to their scalability and rapid growth rates with low endotoxin levels, they are the host cell line of our choice. Further, the use of CHO cells enable us to produce recombinant antibodies animal-free.
If you are looking for large scale protein expression for drug development or smaller scale expression for research purposes, evitria offers scalable solutions that fit your project’s needs and is ready to support you from generating antibodies to purification and shipment.