Recombinant describes DNA, proteins, cells, or organisms that are created by combining genetic material from two different sources in order to alter their characterization. They are then tested in clinical trials and used in different healthcare and biotechnology products, such as mrna vaccines.
The synthetic genes used for recombinant antibodies are typically generated from a plasmid or from an integrated sequence in a stable cell line. Expression takes place in host cells, with the aim to alter the material and achieve translational modifications.
Recombinant DNA technology describes a process of genetic engineering that uses enzymes and various laboratory techniques to isolate and manipulate genetic material. There are different ways to carry out manipulation in an organism’s genome, all with the aim to improve certain characteristics, for instance to achieve greater stability of human proteins, but also to reduce toxicity on normal cells and the associated side effects.
Recombinant monoclonal antibodies, for instance, are produced in vitro (respectively ex vivo), by cloning synthetic genes that have been recovered from monoclonal antibodies, generally from mammalian cells. They are then modified by means of genetic engineering to improve their specificity and reproducibility, as well as antigen binding properties.
Subscribe to our Newsletter
Get all the latest updates, and learn about our advancements in antibody production.
Subscribe now
Recombination is used in the biotechnology sector to overcome weaknesses of polyclonal and traditional monoclonal antibodies in terms of quality and reproducibility. It serves several functions in organisms, including DNA repair in bacteria and eukaryotes, and ensures the correct alignment and segregation of chromosomes during meiosis in eukaryotes.
Technologies to alter genetic material have been successfully applied to generate important proteins used in diagnostics, clinical trials and general health care.
Improved specificity and a favorable replication factor are two of the reasons recombinant reagents are increasingly popular:
Recombinant proteins are useful tools in understanding protein-protein interactions. They allow scientists to study protein interactions with other proteins or peptides, enzymes, small molecules, lipids, and nucleic acids. The choice of the host cell whose protein synthesis machinery will produce the protein will initiate the outline of the whole process and also impact the growth factor.
Recombinant DNA molecules, generated for the first time half a century ago, are increasingly used in pharmaceutical and medical fields thanks to a number of benefits, such as their reliable expression and distribution as DNA sequences and plasmids.
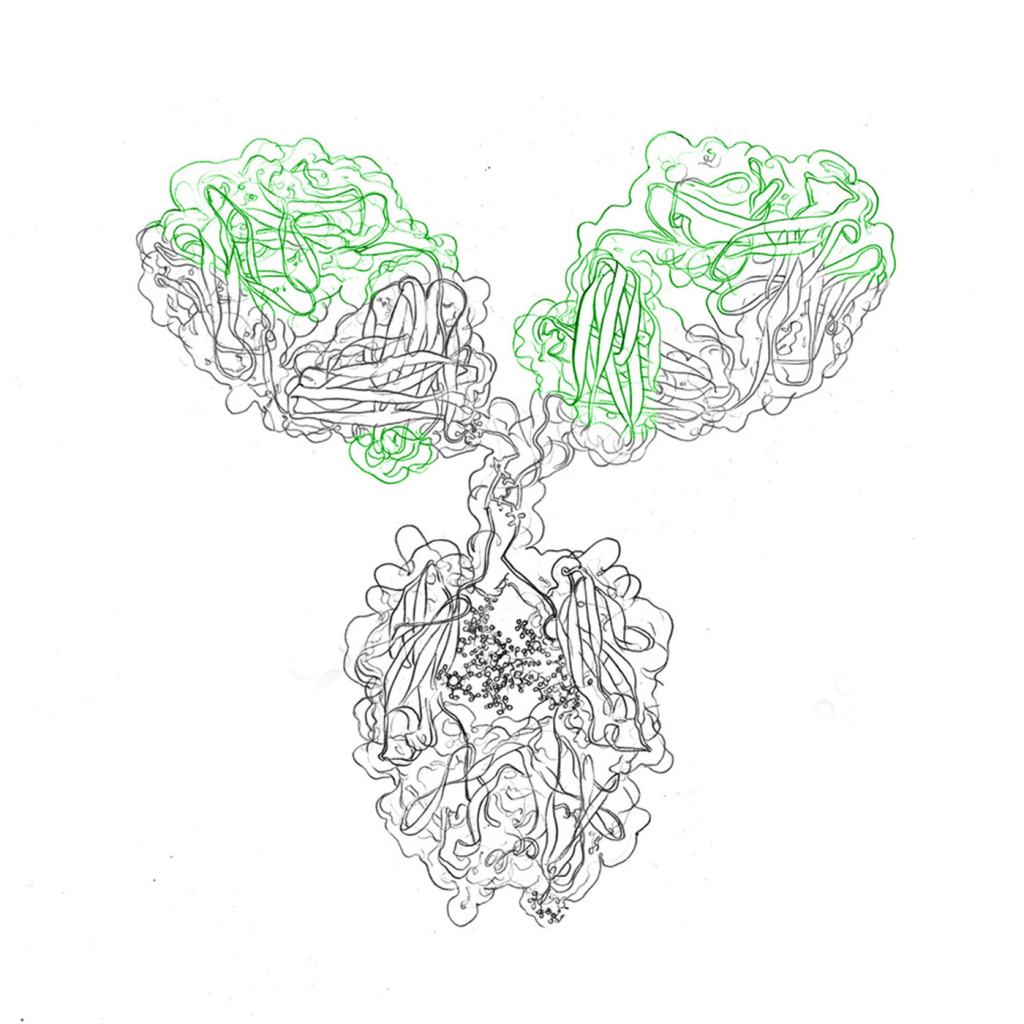
Recombinant antibodies have multiple uses, e.g. as valuable diagnostic research tools, like immuno-assays. They are used to study gene expression profiles or for therapeutic protein production. Once the peptide sequence of an antibody is known, it can be modified to fit various needs, making it an ideal candidate for vaccines and other health care products.
According to citations in publications by the National Center for Biotechnology Information and various institutions, to date several hundreds of products based on recombinant protein have been approved by the FDA and NIH. By the way: Human insulin fabricated in the bacterium E. coli was the first biopharmaceutical based on recombinant DNA approved by the FDA – as early as 1982.1
At evitria, recombinant monoclonal antibodies are expressed using mammalian cell lines rather than E. coli cells. Due to their similarity to the human cell system, CHO cells are ideal for genetic studies, toxicity screening and gene expression.