With extensive expertise and experience in recombinant antibody production, evitria offers rapid and efficient bispecific antibody expression services using a state-of-the-art CHO cell expression platform.
This approach ensures the highest quality results and allows us to initiate production from antibody sequences and complete the process in just 4 weeks.
We support the expression of all common bispecific formats, including the Duobody system (Genmab), IgG-scFvs, Knobs-into-Holes, Crossmab, Electrostatic Steering, and other related formats.
Unsure on what bispecific format you should use in your research? We can use our expertise to guide the design of your constructs – come to us with mAbs, and leave with bsAbs through our bispecific screening programme.

Antibody production runs
Antibodies purified
Projects completed on time
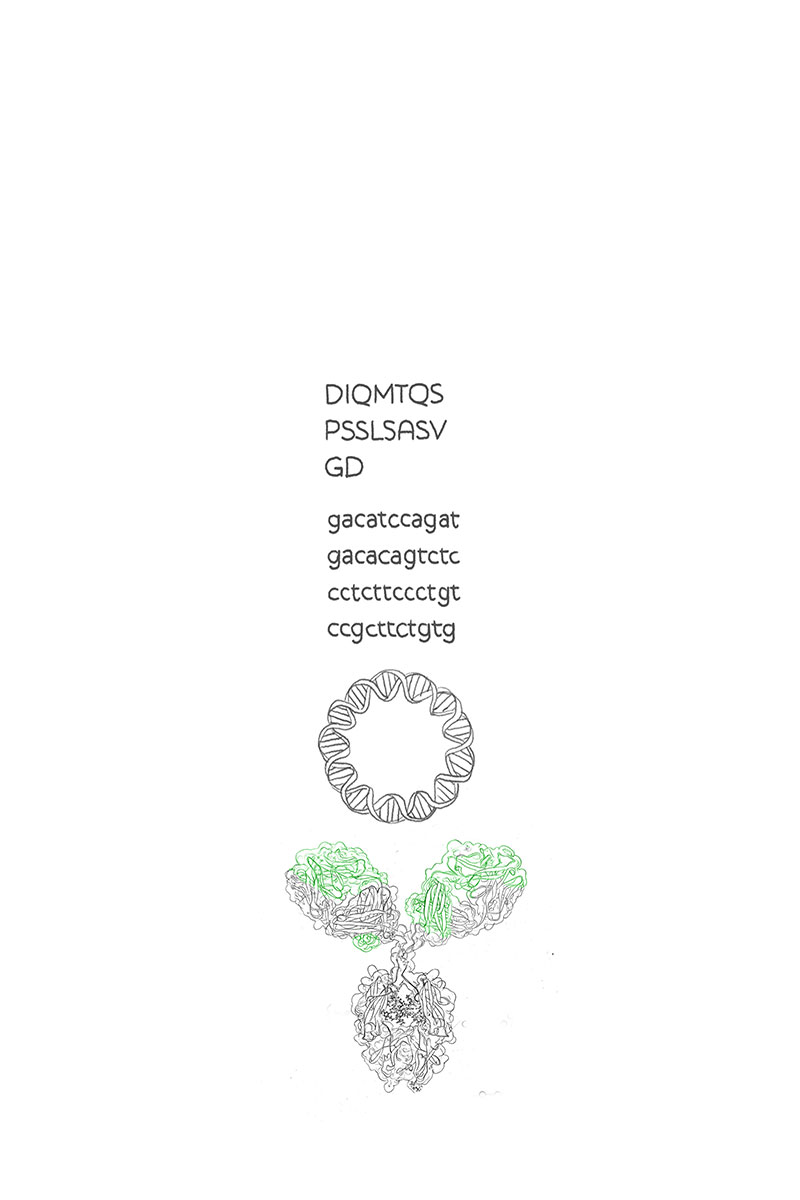
From Sequence to Antibody
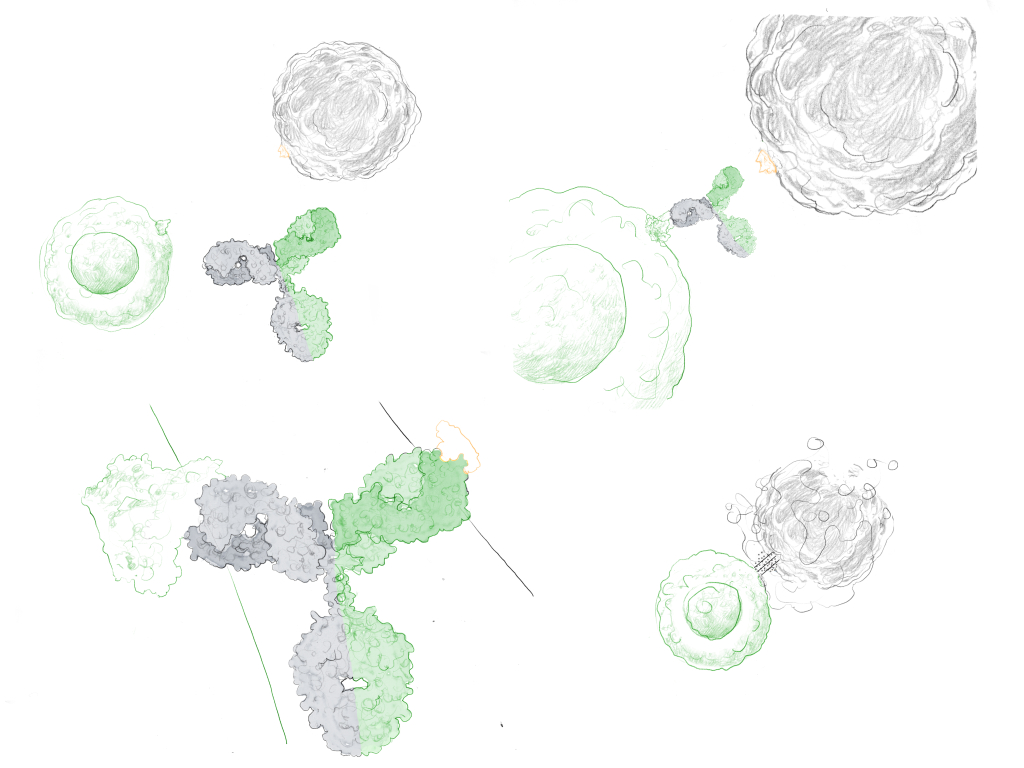
Recombinant antibodies have revolutionized modern therapeutic practices, and in the past 10 years the use of bispecific antibodies – proteins capable of engaging two target antigens simultaneously – has become a key focus area for the industry.
Bispecific antibodies (bsAbs) can be categorized by their activity or by their structural format.
By way of activity, trans co-engagers interact with two different antigens on two different cells, for instance, bringing a T-cell into close proximity with a tumor cell and activating cytotoxic pathways for cell destruction (Figure 1). Cis co-engagers interact with two different antigens on the same cell, thereby improving the selectivity of the antibody and reducing therapeutic side effects.
Structurally, antibodies can be categorized based on the number of binding domains which they possess (their valency) and how they are divided between the different targets. For instance, bispecific antibodies are often classified as “1 + 1” (two binding sites, one for each target), “2 + 2” (four binding sites, two for each target), or “2+1” (three binding sites, two for one target, one for the other). Further combinations are possible, and more targets can be engaged using multi-specific antibodies.
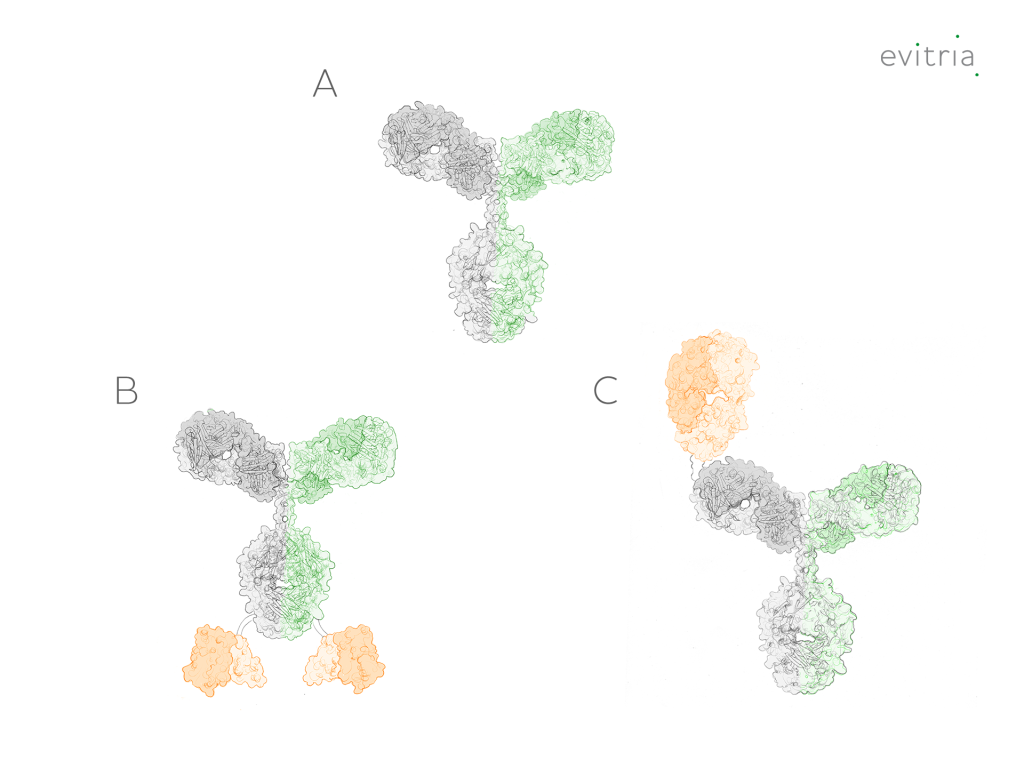
Additional structural characterization can be made by looking at the format of the bispecific and the sequences used to join the different domains. “Traditional” structures closely resemble regular monoclonal antibodies (mAbs) but have different binding domains on each Fab arm (Figure 2A). These can be further embellished by the addition of other binding regions, such as scFvs or Fab domains, which leads to the creation of multi-specific molecules (Figures 2B and 2C).
Non-antibody bispecifics can be created through the linkage of binding domains without Fc regions. These domains can be antibody fragments, such as individual scFvs (common with bispecific T-cell engagers), Fab domains, VHH domains, or other proteins with an affinity for the desired target(s).
Finally, additional modifications may be added to augment the function of the bispecific molecule. Most frequently, these modifications would be used to silence immune effector functions, which are typically undesirable for trans co-engagers. However, any other mutations used with traditional antibodies can theoretically be transferred into a bispecific antibody.
The choice of which bispecific format to use is non-trivial and should be made through a combination of application, manufacturing, and commercial considerations.
Join the presentation of Desmond Schofield at PEGS Boston
Case Study on Bispecific Antibody Production
Engineering and reformatting your antibody: from mAb to bsAb, PEGS Boston, Tuesday, May 14, 4:00 pm
Contact us in advance
At evitria AG, we have produced over 20,000 different antibodies and antibody-related molecules using our transient CHO cell expression system, and have covered all the major bispecific formats as well as the less used, proprietary, highly engineered, or unusual constructs.
We work with our clients to understand their goals and can provide expert guidance on which formats with which preferred variable domains may be worth pursuing. Your selection(s) will likely come down to a choice between a few main formats described below and subsequent decisions on mutations and/or embellishments.
This proprietary format is often the easiest way to create a bispecific antibody. Here, two individual IgG1 antibodies with complementary CH3 mutations are expressed separately. Following purification, the antibodies can be combined in a process known as Fab arm exchange. This creates a 1 + 1 antibody as shown in Figure 2A.
Expressing individual IgG1 antibodies ensures high yields (the mutations have no impact on expression), and the Fab arm exchange reaction is a relatively straightforward and established procedure. The commercial use of this technology requires a license from Genmab, and evitria AG can support your exploration of this format during the research phase.
This is a 2+2 format, where an scFv is added to the C-terminus of each antibody heavy chain, usually by connection with a flexible linker. The expression and purification of this format is variable and heavily depends on the specific scFv sequence used. Therefore, a SEC polishing is often required for high purities. Overall, this format is often worth evaluating, especially as there are no IP restrictions on its use.
These 1+1 bispecifics are produced during the mammalian cell culture processby transfecting the cells with 2 different heavy chains and one or two light chains. Their behavior is highly sequence-specific, and they often produce homodimers which require specialist purification to separate from the desired heterodimer. Optimization of transfection ratios can improve heterodimer formation but does not guarantee success.
Bispecifics produced using this 1+1 format can then only be purified by ion exchange, which requires a custom-developed process for each candidate. Further, the difference in pI between the homodimer and heterodimer is relatively small, and so the process can be difficult to optimize, scale, and costly to research.
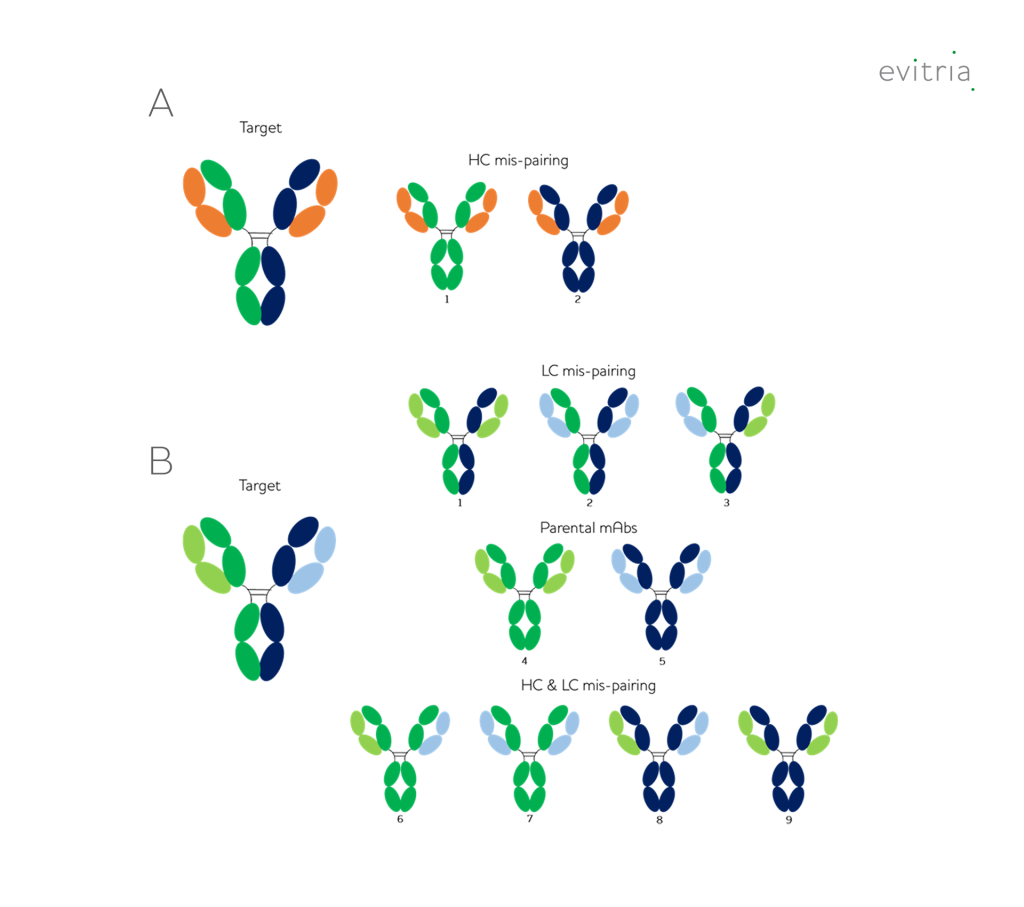
The basic mutation set for Knobs-into-Holes (KIH) bispecifics is available without IP restrictions. However, there are many mutations to the heavy chain constant region described in literature that can reduce or prevent homodimer formation. These mutations are often protected by patents and require licences to commercialize.
A common light chain approach is strongly preferred for use with KIH bispecifics as the addition of a second chain significantly increases the number of incorrect pairings (Figure 3), but further proprietary mutations (such as Crossmab) have been described which can minimise this issue.
From sequence to antibody in 4 weeks
Project start
0-1 business days
Pilot study
(from electronic sequence to quantified pilot supernatant)
10-15 business days
Larger-scale expression
5-10 business days
Purification
2-3 business days

Final analytics
2-3 business days
Shipment
1-2 business days

The format options for creating bi- and multi-specific antibodies are constantly increasing, and with them comes an ever-expanding list of considerations and trade-offs to evaluate. Regardless of where your research is taking you, evitra AG will be available to support and supply you with high quality material, time and time again.
Desmond Schofield is our expert for any questions related to bispecific antibodies. With his doctorate in biochemical engineering from University College London and extensive experience in various fields of biotechnology, including synthetic biology and immuno-oncology, Desmond brings a wealth of knowledge and expertise to evitria. He is dedicated to helping you navigate the complexities of bispecific antibody research and production.
Bispecific antibodies are engineered antibodies designed to bind to two different antigens simultaneously. This dual targeting capability can enhance therapeutic effects, such as bringing immune cells closer to cancer cells to boost immune response.
Below is a comprehensive table of all FDA-approved bispecific antibodies, showcasing the range and potential of these groundbreaking treatments.
| Therapeutic | Bispecific Format | Targets | Year of Approval | Company |
| Amivantamab | Duobody | EGFR x MET | 2021 | Janssen |
| Blinatumomab | Tandem scFv (Bispecific T-cell engager) | CD19 x CD3E | 2014 | Amgen |
| Cadonilimab | IgG-scFv | PRDCD1 x CTLA4 | 2022 | Akeso |
| Emicizumab | Common light chain + Electrostatic Steering | F9 x F10 | 2017 | Genentech |
| Faricimab | Crossmab Knob-in-Hole | VEGFA x ANGPT2 | 2022 | Roche |
| Mosunetuzumab | Knob-in-Hole | CD20 x CD3E | 2022 | Genentech |
| Tebentafusp | ImmTAC (Immune Mobilizing Monoclonal TCRs Against Cancer) | GP100 x CD3E | 2022 | Immunocore |
| Teclistamab | Tandem scFv | BCMA x CD3E | 2022 | Janssen |
| Epcoritamab | Duobody | CD20 x CD3E | 2023 | Genmab |
| Glofitamab | Two-chain scFv (2:1 format) | CD20 x CD3E | 2023 | Roche |
Multispecific antibodies are engineered antibodies that can bind to multiple antigens at once. They are designed to engage various targets for more precise and effective treatments, offering broader therapeutic options and potential for complex diseases.
Bispecific antibodies are successfully applied in various therapeutic areas such as oncology, immunology, and hematology. They are particularly effective in immunotherapy, where they can recruit immune cells to target cancer cells.
Bispecific antibodies can improve treatment efficacy by engaging two targets simultaneously, potentially leading to stronger immune responses and increased precision in targeting diseases. They also offer flexibility in designing tailored treatments for complex conditions.