Custom recombinant antibody production
We are a leading service provider for custom recombinant antibody production. Get in contact!
Bispecific antibodies (bsAbs) have emerged as a groundbreaking class of biopharmaceuticals, revolutionizing the field of antibody therapeutics and diagnostics. These unique molecules possess two distinct binding sites, allowing them to simultaneously engage with multiple antigens, effector cells, or disease targets. This exceptional versatility opens up a wide range of therapeutic and diagnostic possibilities, making bispecific antibodies a promising avenue in cancer therapy, immunotherapy, and various other disease areas.
In this comprehensive overview, we delve into the field of bispecific antibody production, exploring their definition, relevance, types, and structures. We will unravel the intricate processes involved in their production, with a focus on recombinant antibody techniques and optimization strategies.
Bispecific antibodies are remarkable and innovative molecules engineered to possess two distinct binding sites, combining the binding specificity of two different monoclonal antibodies. These antibodies enable simultaneous targeting of multiple antigens, effector cells, or disease targets, presenting exceptional opportunities in various therapeutic and diagnostic applications.1
Bispecific antibody formats can include recombinant single-chain variable fragments (scFv), Fab fragments, or IgG-like bispecific antibodies. The optimization of bispecific antibodies involves techniques like knobs-into-holes mutations to improve their specificity and binding affinity. These unique attributes make bispecific antibodies powerful tools in cancer treatment, immunotherapy, and other areas of biomedical research.
Subscribe to our Newsletter
Get all the latest updates, and learn about our advancements in antibody production.
Subscribe now
Bispecific antibodies have gained significant relevance in the field of biotechnology and medicine due to their exceptional versatility and unique mechanisms of action. Unlike traditional monoclonal antibodies that target a single epitope, bispecific antibodies can simultaneously engage with different epitopes on target antigens or multiple cell surface receptors. This dual targeting ability provides distinct advantages in various therapeutic and diagnostic applications.
In cancer therapy, bispecific antibodies and fragments (such as bispecific t-cell engagers) offer the potential to target specific antigens on tumor cells while engaging with immune effector cells, such as T-cells or natural killer (NK) cells, to trigger targeted immune responses against leukemia, solid tumors, and other malignancies. By recruiting effector cells to the tumor site, bispecific antibodies can enhance the efficacy of immune-mediated cytotoxicity, leading to improved therapeutic outcomes.
Furthermore, bispecific antibodies can bridge the gap between b-cells and specific antigens, facilitating the targeting of disease-causing agents or pathogenic cells. Their unique structures, such as dual variable domain antibodies and CrossMab formats, enable precise binding and interaction with various disease-associated targets, resulting in enhanced therapeutic potential.
In addition to therapeutic applications, bispecific antibodies are also employed in diagnostic assays, allowing the simultaneous detection of multiple disease markers. This capability aids in the accurate identification and profiling of specific antibodies or antigens, enabling early disease diagnosis and personalized treatment strategies.
The ongoing clinical trials and successful implementation of bispecific antibodies in various biologics underscore their significant relevance in the rapidly evolving landscape of therapeutic antibodies. Their ability to harness multiple pathways, trigger effector functions, and address complex diseases with specific targeting make them promising candidates for next-generation biologics and therapeutic antibodies. As researchers continue to explore and optimize their properties, bispecific antibodies hold tremendous potential in shaping the future of precision medicine and advancing novel therapeutic strategies for various diseases.
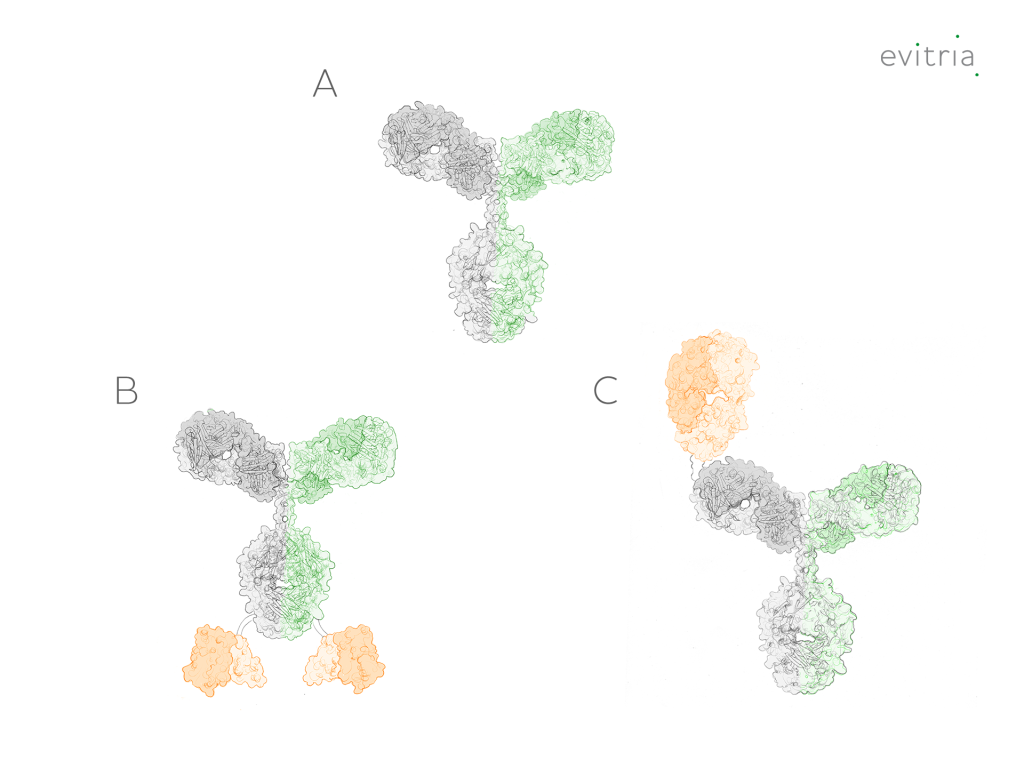
Bispecific antibodies come in various types, formats, and structures, offering a wide range of tools for targeted therapeutics and diagnostics. The diverse antibody engineering strategies employed to create bispecific antibodies result in unique functionalities tailored to specific applications.
Bispecific antibodies are created through sophisticated antibody engineering techniques. These methods involve fusing two distinct monoclonal antibodies (mAbs) or antibody fragments, resulting in bispecific formats with dual binding specificities. Commonly used formats include scFv (single-chain variable fragments) and Fab fragments, which retain antigen-binding regions from parent antibodies.
To connect the two binding sites, various linker technologies are employed. The linker ensures stable bispecific antibody formation and may influence their pharmacokinetics and effector functions. Different linker designs optimize the balance between flexibility and stability.
As a result of the diverse processing steps, different bsAb variants can be manufactured. Some important types are:
The diverse types, formats, and structures of bispecific antibodies continue to expand, driven by ongoing research and technological advancements.
We are a leading service provider for custom recombinant antibody production. Get in contact!
The production of bispecific antibodies involves sophisticated protein engineering and advanced production techniques. Here’s an overview of the key aspects in their production process:
Bispecific antibody production is a complex and intricate process, necessitating cutting-edge protein engineering and purification techniques. The ability to generate bispecific antibodies with desired functionalities, including prolonged half-life (e.g. IgG1 format) and asymmetric structures, is a cornerstone in their continuous improvement.
Ongoing efforts are being made to enhance the efficacy and applicability of bispecific antibodies, aiming to maximize their therapeutic potential and reduce potential challenges. Several strategies are employed to improve the properties of bispecific antibodies.
Improving the binding specificity of bispecific antibodies is critical to avoid off-target effects and increase therapeutic selectivity. Advanced antibody engineering techniques, such as site-directed mutagenesis and rational design, enable precise control over antibody-antigen interactions.
Aggregation is another common challenge in bispecific antibody development that can compromise stability and bioactivity. Innovative formulation and purification methods, including chromatographic and filtration techniques, are employed to minimize aggregation and ensure consistent product quality.
Immunogenicity, the potential for antibodies to induce an immune response, can impact the efficacy and safety of bispecific antibodies. By optimizing the amino acid sequences and employing humanized frameworks, researchers aim to reduce immunogenicity and improve in vivo tolerance.
Moreover, modulating the half-life of bispecific antibodies is essential to optimize their pharmacokinetics and therapeutic efficacy. Incorporating Fc region modifications or employing half-life extension technologies helps to tailor the antibody’s persistence in vivo, providing sustained therapeutic benefits.
Bispecific antibodies have shown significant promise in cancer immunotherapy, especially in targeting cancer cells while engaging immune cells to initiate anti-tumor immune responses. Continuous research and clinical development are focused on optimizing bispecific antibodies for potent and selective cancer cell targeting.
Rigorous in vitro and in vivo assays are employed to evaluate the functional characteristics and potential side effects of bispecific antibodies. These assays provide critical insights into their efficacy, safety, and mechanisms of action.
Considering the complexity of the manufacturing process of bispecific antibodies, several biopharmaceutical companies and research teams outsource the production of bispecific antibodies to CDMOs. Nevertheless, this process requires not only profound expertise, but also consumes a high amount of resources.
evitria can provide both thanks to our profound experience in transient recombinant antibody production services. With a high focus on individualization, we can produce several bispecific antibody types that meet the high standards of our partners and regulatory requirements.