Therapeutic antibodies stand at the forefront of modern biomedicine. These remarkable molecules, designed to target specific antigens, hold immense promise in the treatment of various diseases. In this article, we discover the mechanisms of antibody-dependent cellular cytotoxicity (ADCC) and explore the technique of afucosylation. Additionally, we’ll highlight the role of companies like evitria in spearheading the production of afucosylated antibodies, contributing to the evolution of therapeutic treatments.
Therapeutic antibodies are the fastest-growing class of biological drugs. Their ability to interact with specific targets means they can be employed for a range of diseases, including cancer, autoimmune and infectious diseases. According to the Umabs Antibody Therapies Database, 162 antibody therapies had gained approval by at least one global regulatory body at the end of June 20221.
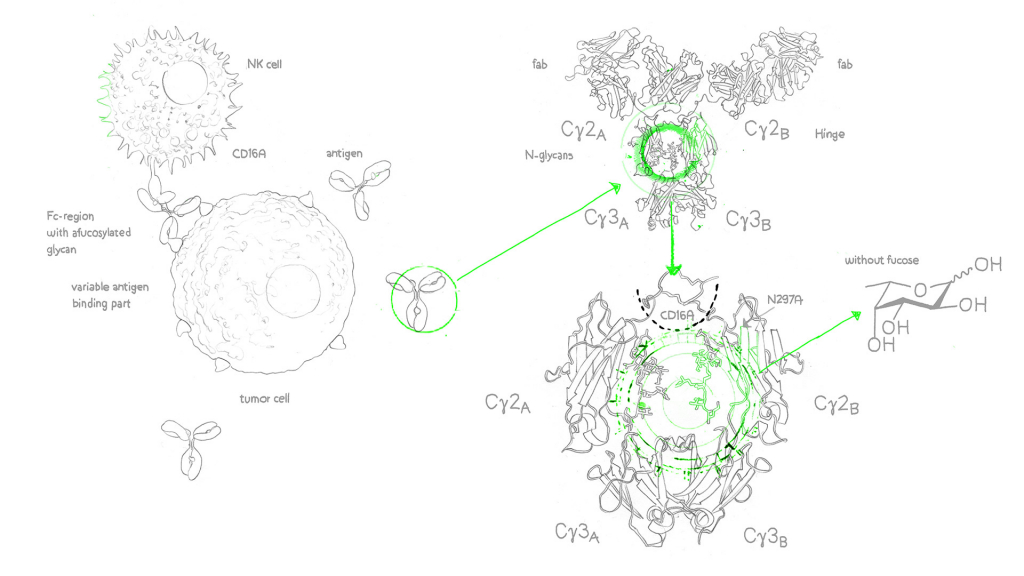
One critical mechanism underlying the clinical efficacy of therapeutic antibodies is antibody-dependent cellular cytotoxicity (ADCC). Although several classes of antibodies can mediate ADCC, most therapeutic antibodies belong to the IgG subclass. ADCC relies on the bifunctional structure of antibodies, where the antigen-binding fragment (Fab) portion recognises an antigen, and the fragment crystallisable (Fc) portion bridges the antibody to effector cells possessing Fc receptors (FcγR).
FcγRs are glycoproteins expressed on effector cells, like natural killer cells (NK), neutrophils and eosinophils. The typical ADCC involves the activation of NK cells following the binding of its FcγRIIIa (CD16a) to the Fc portion of IgG antibodies. This causes polarisation of cytotoxic granules and releases perforins and granzymes, which work in concert to induce cell death. In targeted cancer therapy, antibodies such as rituximab, trastuzumab and cetuximab increase tumour-cell killing via ADCC.
Subscribe to our Newsletter
Get all the latest updates, and learn about our advancements in antibody production.
Subscribe now
Recently, antibody engineering techniques have focused on enhancing ADCC to provide more efficacious antibody treatment. Glycoengineering involves removing the fucose residues on the Fc region of the antibody. This strategy targets the N-linked glycosylation site at residue 297 (N297) in the Fc region, which consists of a heptasaccharide core structure that can undergo further extension with glycan moieties, such as fucose.
Removing fucose from the Fc N-glycans of human IgG1 increases the affinity to human FcγRIIIa on NK effector cells by 10-100 fold compared with non-fucosylated counterparts2–4. Structural studies have explained that this increased affinity is due to the strengthening of the carbohydrate-carbohydrate interactions between the antibody Fc region and the FcγRIIIa on effector cells. As FcγRIIIa is a major activating receptor on NK mediating ADCC, this leads to a 2-40 fold increase in ADCC3–5. Moreover, afucosylation does not change other properties like antibody conformation and stability6.
Afucosylated antibodies have a clinical advantage because of their enhanced immune activation of effector cells. Examples of clinically approved afucosylated antibodies include:
Afucosylated antibodies with either a complete absence or reduced levels of fucose can be generated using genetically engineered cell lines with defects in enzymes involved in the fucose biosynthesis pathway. Alternative strategies include recombinant fucosidases and post-manufacturing enzymatic treatment. Interestingly, afucosylation has overtaken other strategies — such as Fc mutations with five substitutions (L235V/F243L/R292P/Y300L/P396L) — to become the most popular strategy for enhancing antibody effector functions (Table 1).
| Fc enhancing variants | Number of INNs |
| Reduced levels of fucose (achieved by host cell engineering) | 10 |
| E430G | 3 |
| L235V/F243L/R292P/Y300L/P396L | 2 |
| S239D/K274Q/Y296F/Y300F/L309V/I332E/A339T/V397M | 2 |
| V215A | 2 |
| G236A/A330L/I332E | 1 |
| G236A/S239D/A330L/I332E | 1 |
| N325S/L328F | 1 |
| P247I/A339Q | 1 |
| S239D/A330L/I332E | 1 |
| S239D/I332E | 1 |
| S267E | 1 |
| S267E/L328F | 1 |
| T393A | 1 |
| V215A/E269R/K322A | 1 |
evitria is a global antibody expression service provider for afucosylated antibodies. evitria efficiently expresses afucosylated antibodies lacking the core fucose moiety by utilising ProBioGen’s GlymaxX® technology. For this, the heterologous expression of the bacterial oxidoreductase GDP-6-deoxy-D-lyxo-4-hexulose reductase (RMD) deflects an intermediate in the de novo synthesis of fucose to a dead-end product10, resulting in the secretion of afucosylated antibodies. As part of the service, evitria can validate each antibody by assessing the ratio of G0/G0F using mass spectrometry (Figure 1) to calculate the percentage of afucosylation.
evitria excels in the transient expression of native and afucosylated antibody variants in Chinese hamster ovary (CHO) cells using the GlymaxX® technology. Afucosylated antibodies are produced at the same yields and possess the same stability as native antibodies, thus providing the perfect negative control. Unlike strategies using Fc mutations, evitria’s use of GlymaxX® avoids the risk of immunogenicity. Additional benefits of evitria’s afucosylated antibody service compared with alternative strategies are avoiding inefficiencies caused by steric hindrances of recombinant fucosidases or expensive post-manufacturing enzymatic treatment.
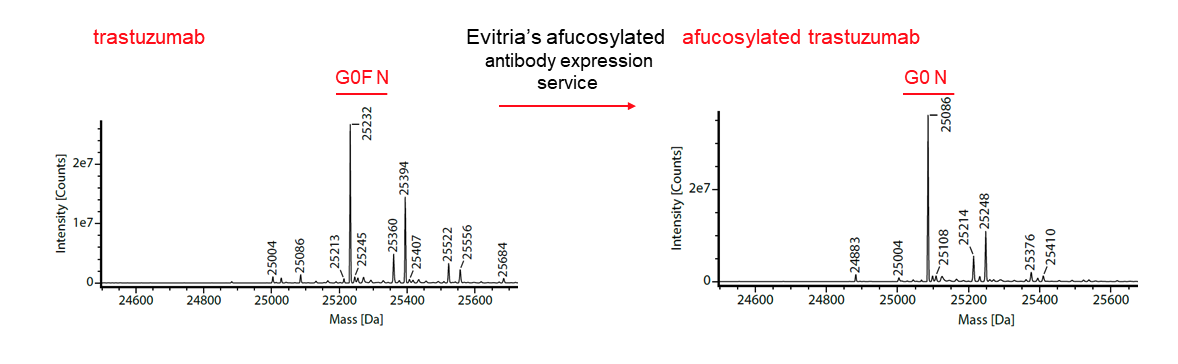
Percentage afucosylation is calculated by comparing fucosylated (G0F) and afucosylated (G0) antibody forms.
evitria has aided in the preclinical development of afucosylated antibodies for treating T-cell acute lymphoblastic leukaemia (T-ALL). Classed as an orphan disease (lacking immunotherapeutic options), T-ALL is a rare, aggressive form of leukaemia caused by the lack of proper development of T-cells, leaving them unable to fight infections properly. CD43 is highly expressed in T-cells. In a study published in the Journal for Immunotherapy of Cancer, researchers investigated whether an afucosylated humanised antibody targeting a unique epitope of CD43 (UMG1) — produced by evitria — could treat T-ALL.
The afucosylated UMG1 antibodies proved effective with significant ADCC against T-cells in vitro. In a leukaemia cancer mouse model, the afucosylated antibodies also exhibited treatment benefits with improved survival rates11.