Custom recombinant antibody production
We are a leading service provider for custom recombinant antibody production. Get in contact!
The development of afucosylated antibodies implies a modification process altering the structure of antibodies, and involves the removal of a specific sugar molecule called fucose from the antibody’s carbohydrate chain. This modification significantly impacts the antibody’s functionality and interactions within the immune system. By eliminating fucose, afucosylated antibodies exhibit altered behavior, particularly in their ability to engage immune cells and potentially enhance their effectiveness in specific biological activities.
In this article, we will explore the methods and techniques used in afucosylation, shedding light on the science behind this crucial alteration in antibody structure.
Afucosylation of antibodies offers distinct advantages, primarily enhancing their ability to engage with the immune system. By removing fucose, afucosylated antibodies exhibit heightened antibody-dependent cellular cytotoxicity (ADCC).
This modification augments their capacity to bind more efficiently to immune cells, potentially leading to increased destruction of targeted cells or pathogens. Consequently, afucosylated antibodies hold the promise of significantly bolstering therapeutic efficacy, paving the way for more potent and targeted treatments against various diseases.
Subscribe to our Newsletter
Get all the latest updates, and learn about our advancements in antibody production.
Subscribe now
Creating afucosylated antibodies involves altering their structure by targeting the sugar components within the Fc region. This modification primarily focuses on reducing or eliminating fucose residues.
Read more: Applications of afucosylated antibodies
Scientists employ genetic engineering, enzymatic modification, or glycoengineering techniques to achieve this alteration. The goal is to manipulate the glycan structure to minimize fucose content, enhancing the antibodies’ functional properties.
There are different, very specific methods in afucosylated antibody development, some of which are listed below:
We are a leading service provider for custom recombinant antibody production. Get in contact!
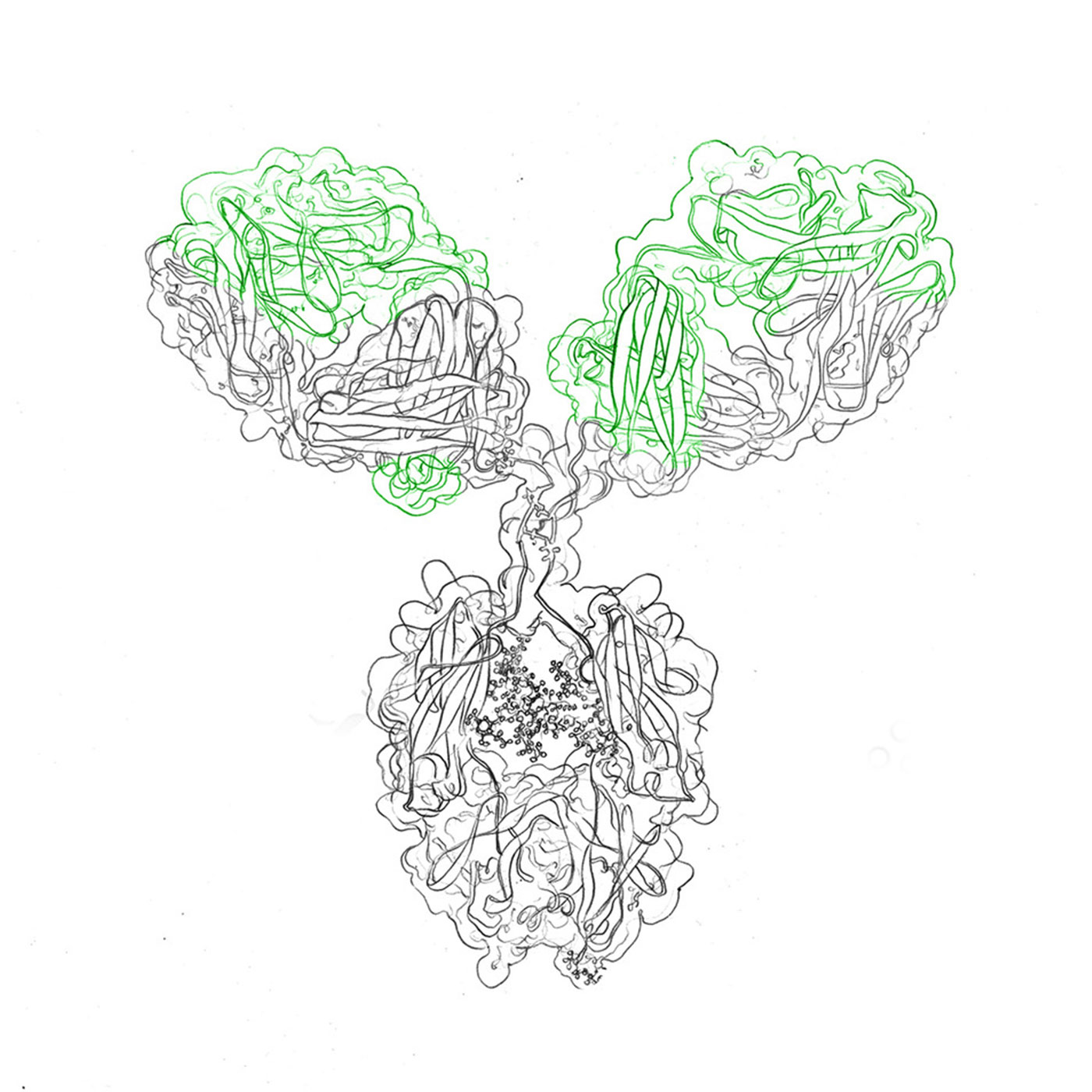
evitria, in an exclusive partnership with ProBioGen’s GlymaxX® technology, offers high quality expression of afucosylated antibodies. Engineered for heightened Antibody-Dependent Cellular Cytotoxicity (ADCC), these antibodies demonstrate superior efficacy against tumorigenic and infected cells.
evitria’s approach, avoiding Fc mutations, ensures equivalent yields and stability to native antibodies with reduced immunogenic risks. Specializing in recombinant antibody production, evitria guarantees swift, high-quality results of afucosylated antibody expression. From sequence to antibody in just five weeks, evitria’s commitment is to timely support partners worldwide with high-quality afucosylated antibodies.