Afucosylated Antibody Expression
Learn more about our afucosylated antibody expression service
Afucosylation is a central procedure to alter the composition of antibodies by lowering their fucose levels. This, on the other hand, has far-reaching consequences on their characteristics and possible applications.
One key focus is afucosylated antibodies and their role in immunotherapy. We will discuss how these antibodies enhance antibody-dependent cellular cytotoxicity (ADCC) and their potential in cancer treatment. Additionally, we will explore the approaches employed to produce afucosylated antibodies, such as glycoengineering and cell line engineering.
Afucosylation is a biochemical phenomenon that involves the alteration of glycoproteins by the removal of fucose residues from their carbohydrate structures.
Fucose is a monosaccharide (a simple sugar) that can be found in various glycans, which are sugar molecules attached to proteins. The absence of fucose residues on certain glycoproteins can have significant biological implications, especially in the immune system.
Subscribe to our Newsletter
Get all the latest updates, and learn about our advancements in antibody production.
Subscribe now
Afucosylation primarily concerns the complex-type N-glycans on glycoproteins. These glycoproteins are often involved in cell signaling, immune responses, and various cellular processes.
Learn more on glycosylation of proteins
One of the most noteworthy consequences of afucosylation is its effect on antibodies. Antibodies, also known as immunoglobulins, play a crucial role in our immune system by recognizing and binding to specific antigens, such as pathogens or abnormal cells. This includes B-cell activation and response. The removal of fucose from the antibody’s glycan structure results in what are known as afucosylated antibodies.1
ADCC is a powerful immune response mechanism employed by the body to combat infected or abnormal cells, such as cancer cells. It relies on a well-coordinated interplay between antibodies, immune cells, and target cells:
Antibodies’ Role: When the body recognizes an invader like a cancer cell, it produces antibodies that can specifically bind to proteins on the surface of the target cell. These antibodies act as markers, flagging the target cell for destruction.
Immune Cell Engagement: Specialized immune cells, such as Natural Killer (NK) cells, possess receptors known as Fc receptors. These receptors bind to the tail (Fc region) of antibodies attached to the target cell.
Cell Destruction: Once engaged with the antibodies, NK cells unleash their cytotoxic abilities. They release toxic substances, such as perforin and granzymes, which create holes in the target cell’s membrane, ultimately leading to its destruction.2
Afucosylated antibodies are monoclonal antibodies (mAbs) designed with an engineered Fc region that lacks fucose sugar units in its oligosaccharides. The majority of approved afucosylated antibodies belong to the IgG1 (immunoglobulin G 1) isotype. Their modification involves the reduction or elimination of a sugar molecule known as “core fucosylation” from the Fc portion of the antibody. This specific alteration has significant implications for the antibody’s function, particularly in its interaction with immune cells.
The absence of core fucosylation in afucosylated antibodies significantly enhances their affinity for Fc gamma receptors on immune cells, including macrophages and natural killer cells. This high affinity translates into a notably more potent and efficient immune response.3
This enhanced binding affinity, a result of reduced core fucose content, underscores the pivotal role of afucosylated antibodies in boosting the ADCC process. It enables the antibodies to bind more tightly to Fc receptors on immune cells, particularly NK cells, which mediates a more efficient recruitment of immune cells to identify and eliminate abnormal cells.4,5
Afucosylation, as a biochemical modification, exerts a notable influence on various biological processes, particularly in the realms of cell signaling and immune responses.
In the intricate web of cellular communication, glycoproteins act as essential messengers. They facilitate interactions between cells and their environment, orchestrating a wide range of physiological processes. Afucosylation, by altering the glycan structures of specific glycoproteins, can modulate these signaling pathways.6
Afucosylation’s significance in the immune system is closely associated with the mechanism known as antibody-dependent cell cytotoxicity (ADCC), a critical component of immune responses against pathogens and cancer cells.
As previously mentioned, afucosylated antibodies, with low fucose content, exhibit enhanced ADCC activity. In ADCC, immune cells like natural killer cells (NK) cells are activated when they encounter target cells bound by antibodies. Afucosylated antibodies are more effective at recruiting and activating NK cells, leading to more potent target cell destruction.
Therapies involving afucosylated antibodies have shown promise in enhancing the body’s ability to target and eliminate cancer cells. Afucosylated antibodies recombinant nature allows for precise customization, offering a potent tool to target and combat diseases more effectively.7
Afucosylation plays an essential role in the world of oncology, where it influences the progression and treatment of cancer in several significant ways. Nevertheless, these adaptations in structure lead to optimized antibody products, increasing the advantages of antibodies in cancer therapy, such as:
Now that we know why they are valuable, let’s explore how afucosylated antibodies could be produced:
In this method, scientists manipulate the cells responsible for producing antibodies. By tweaking these cells, researchers can ensure that they generate antibodies with the desired afucosylation.
Alternatively, scientists can directly modify the sugars present on the antibodies themselves. This is achieved by creating a tailored environment for antibody production, one that encourages the addition of sugars devoid of fucose.9
Learn more about our afucosylated antibody expression service
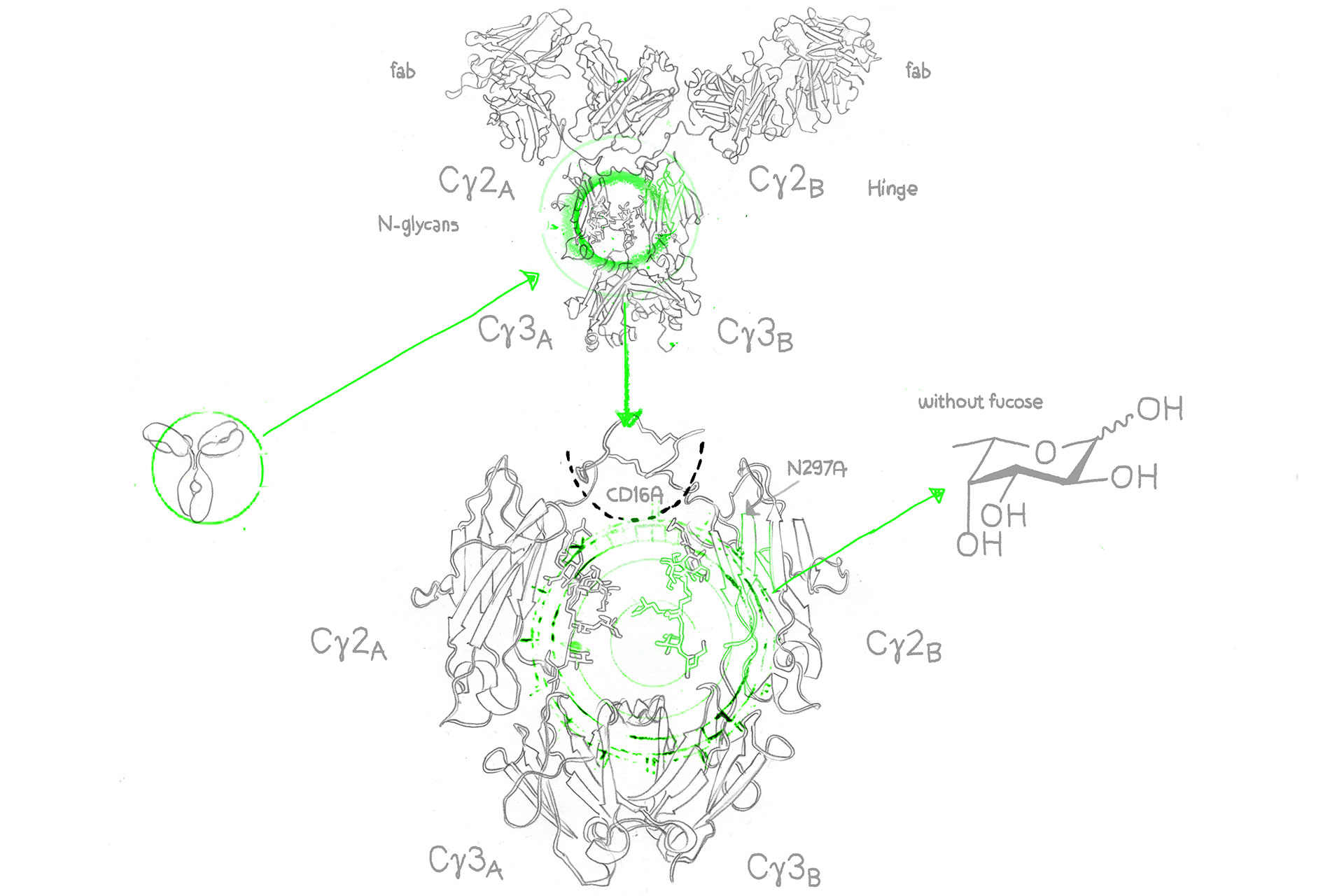
Afucosylation achieved through the GlymaxX® platform is a result of glycoengineering offered by evitria. This innovative approach relies on the GlymaxX® technology from ProBioGen to control the presence of fucose in mammalian cells. Essentially, it uses a unique enzyme that diverts the metabolic pathway of fucose precursors, reducing the availability of fucose monosaccharides for glycosylation processes.
The GlymaxX® technology is built upon a Chinese hamster ovary (CHO cell) expression system known for its genetic stability and reliability, even in large-scale production.
This cutting-edge technology empowers evitria to achieve transient expression of both native and afucosylated antibody variants, without any modifications to the amino acid sequences.
In contrast to alternative afucosylation methods that may entail Fc mutations carrying risks of immunogenicity, evitria’s utilization of GlymaxX® offers equivalent yields and stability as native antibodies.
In summary, afucosylation stands as a novel and transformative development in the realm of biotechnology and immunotherapy. Its ability to enhance immune responses, primarily through the lack of fucose in antibodies, plays a pivotal role in mediating targeted and efficient disease interventions, notably in cancer treatment.
Innovative technologies like GlymaxX® are revolutionizing antibody production, allowing for precise customization and robust characterization in various therapeutic strategies. This novel approach not only holds promise in oncology but also extends its potential to autoimmune and infectious diseases, further underscoring its significance in modern medicine.
Recommended readings from Desmond Schofield:
Afucosylated Antibodies – an introduction
Antibody-Dependent Cellular Cytotoxicity (ADCC) – definition, mechanism & application
Afucosylated Antibody Expression Service
Glycoengineering: pushing forward biotechnology
Afucosylated antibodies are recombinant monoclonal antibodies with reduced fucose content. They play a crucial role in enhancing immune responses, particularly in immunotherapy.
Afucosylated antibodies, with their unique glycoforms, improve immune responses by recruiting immune cells more effectively to target and eliminate harmful cells, such as cancer cells.
Afucosylated antibodies exhibit increased killing kinetics against tumorigenic or infected cells. They are highly valuable in cancer treatment, especially in the context of lymphocytic cancers, as they can precisely target cancer cells while minimizing damage to healthy cells.
Effector functions refer to the antibody’s ability to engage with immune receptors and enhance immune responses, a key feature of afucosylated antibodies.
Core fucose is a sugar molecule on antibodies. Reducing core fucose in afucosylated antibodies enhances their effectiveness in recruiting immune cells.
FcγRIII, or Fc Gamma Receptor III, refers to a family of immune receptors found on the surface of immune cells. These receptors play a crucial role in binding to the Fc region of antibodies, initiating immune responses. FcγRIII includes various FcγR variants, with FcγRIIIa and FcγRIIIb being notable members.
FcγRIIIa, or Fc Gamma Receptor IIIa, is a specific variant within the FcγRIII family of receptors. It is prominently found on immune cells like natural killer (NK) cells, macrophages, and neutrophils. FcγRIIIa plays a pivotal role in enhancing immune responses by binding to the Fc region of antibodies, facilitating processes like antibody-dependent cellular cytotoxicity (ADCC) and phagocytosis.
Yes, afucosylated antibodies are typically produced using recombinant DNA technology in vitro, enabling precise customization and controlled production.
Afucosylated antibodies are not typically naturally produced within a living organism (in vivo). Their production primarily relies on in vitro techniques, such as recombinant DNA technology and cell line engineering. These methods allow for precise control over the antibody’s glycosylation pattern, ensuring the desired afucosylated variants are generated for therapeutic purposes.
Fucosyltransferase is an enzyme responsible for adding fucose sugar molecules to glycoproteins and glycolipids. This modification plays crucial roles in cell signaling, immune responses, and various cellular functions by altering the structure and function of these molecules.
The recommended host cell line for producing afucosylated antibodies is often the Chinese hamster ovary (CHO) cell line. CHO cells are widely used in biotechnology and pharmaceutical industries for the production of therapeutic antibodies. These cells offer stability and reliability in large-scale production processes and can be engineered to achieve the desired glycosylation pattern, including reduced fucosylation.