Antibodies are one of the cornerstones in our immune system, able to detect intruders and foreign substances. By initiating a subsequent immune response, they are essential in the defense against viruses, bacteria and other pathogens.
Due to their unique mechanism of action, the importance of antibodies is not limited to the natural immune response: Biotechnology, for instance, have developed recombinant antibodies as powerful, genetically modified immunoglobulins that can be used in the treatment of several medical conditions.
In this article, we will provide some general information on antibodies – including the definition, structure and mechanism of action of antibodies.
Antibodies (also referred to as immunoglobulins) are specialized proteins produced by immune cells called B cells. They play a crucial role in the body’s defense by detecting pathogens and signalling their presence. But even after exposure, they remain in the blood stream to safeguard the body from a repeated infection.1
Subscribe to our Newsletter
Get all the latest updates, and learn about our advancements in antibody production.
Subscribe now
Antibodies are highly specific, with the ability to recognize and target particular antigens, marking the beginning of the immune response. Their distinct Y-shaped structure is composed of four polypeptide chains, consisting of two pairs of heavy and light chains. The interactions between these chains are stabilized by disulfide bonds, giving antibodies their unique shape.
The interplay of antigen vs. antibody is a fundamental process in immunology, critical for defending the body against foreign intruders.
Antigens, as the initiators, are foreign substances that provoke an immune response. They can be proteins, molecules, or even parts of pathogens. When encountered by the immune system, antigens serve as red flags, signaling a potential threat.
Antibodies, conversely, are the immune system’s specialized responders. They are proteins crafted by immune cells in response to specific antigens. The remarkable attribute of antibodies is their specificity; they possess antigen-binding sites that perfectly complement the structure of a particular antigen.
The connection between antigens and antibodies is akin to a lock and key mechanism. Each antibody is tailored to a specific antigen, with its binding site serving as the key that fits precisely into the lock of the antigen’s structure. Therefore, antibody binding is the immune system’s way of tagging antigens for destruction or neutralization.
Antibodies play a critical role in immunology, being essential for a well-coordinated immune defense. Their operation involves various immune cells and processes.
It all begins with the activation of B cells (a white blood cell type, also referred to as B lymphocytes), responsible for producing antibodies tailored to recognize specific antigens on pathogens. When antibodies encounter these antigens, a series of events unfolds. B cells transform into plasma cells, specialized factories that produce and release antibodies into the bloodstream.
Circulating antibodies seek out and attach to pathogen antigens, effectively tagging these invaders for neutralization. Immune cells like macrophages recognize the tagged pathogens, engulfing and eliminating them. T cells play a crucial role in fine-tuning the immune response by activating different immune cells, ensuring a precise defense against infections.
Antibody structure is key to their unique mechanism of action, therefore vital for their function. Their architecture is typified by a Y-shaped design, constituted by four polypeptide chains – two identical light chains and two identical heavy chains. The region where these chains join contains disulfide bonds, stabilizing the structure.
Within antibodies, two distinct regions are discernible: the constant region (Fc) and the variable region (Fab). The constant region remains relatively consistent, determining the antibody’s class or isotype. In contrast, the variable region exhibits unique characteristics, including the antigen-binding site or epitope, critical for specific antigen recognition.
This structural intricacy enables antibodies to precisely target antigens and engage in a multifaceted immune response, recognizing and binding to foreign substances while triggering immune cell activation through receptor interactions.
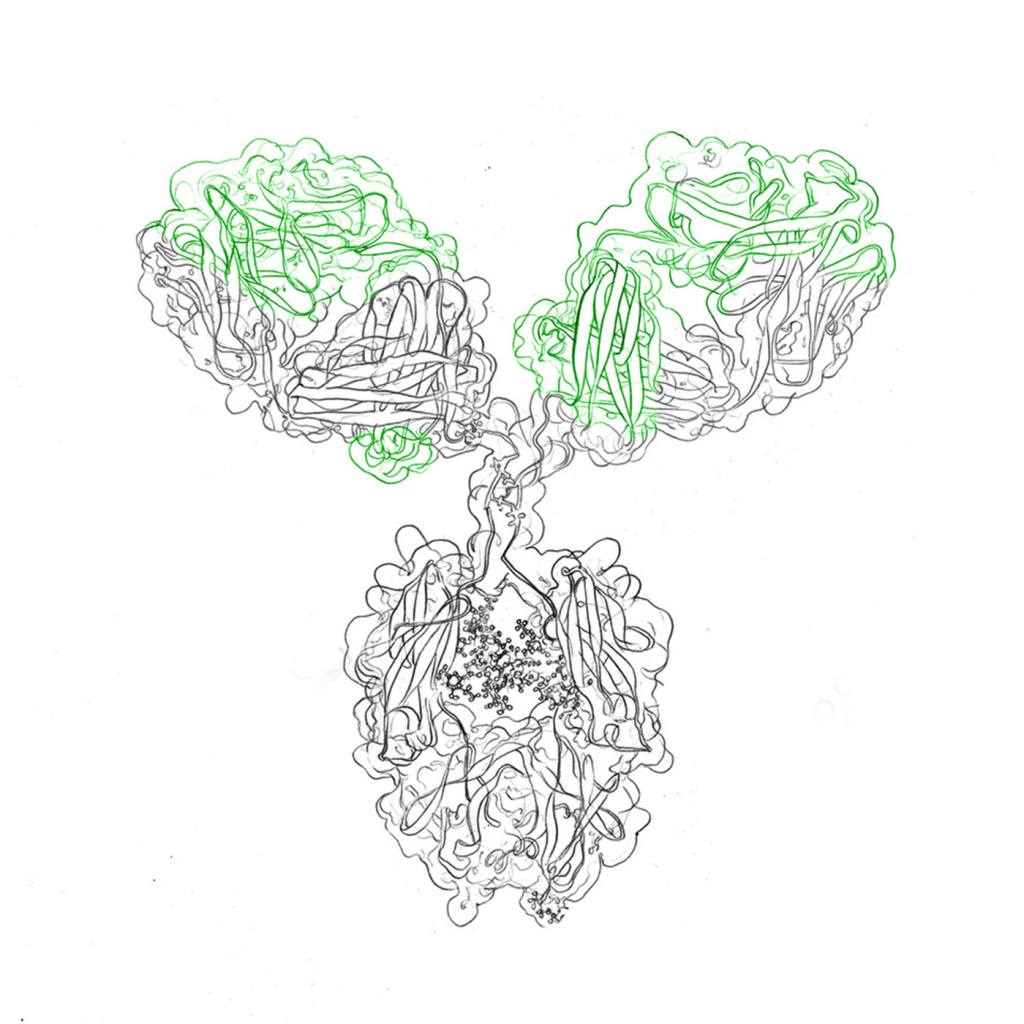
Antibodies are incredibly diverse, categorized into several classes or isotypes, each with distinctive roles in the immune system. Specific antibodies, each characterized by their isotype and determinant recognition, collaboratively bolster the immune system’s versatility and effectiveness.
Among the most common types are IgG, IgM, IgA, IgE, and IgD.
Antibodies are vital tools for healthcare, diagnostics, research, and therapeutics, with two primary modes of production: natural and in vitro.
Inside the body, immune cells, particularly B cells, play a central role in producing in vivo antibodies. When exposed to antigens, B cells generate antibodies tailored to recognize and neutralize the specific threat. This process is fundamental in developing immune responses, both in infections and after vaccination. It also contributes to passive immunity, where maternal antibodies protect newborns. In healthcare, natural antibody production underpins serological tests like ELISA (Enzyme-Linked Immunosorbent Assay) and Western blot, aiding in diagnostics.
Read more: Polyclonal vs. monoclonal antibodies
In vitro, or outside the body, antibody production is achieved through advanced biotechnological methods. Monoclonal antibody production, crucial for precision medicine, is carried out in controlled environments. Recombinant DNA technology allows for the creation of precise antibody structures, offering versatility in applications, such as cancer therapy, where antibodies may target cancer cells with exceptional accuracy by recognizing certain components on their cell surface. These laboratory-produced antibodies also find applications in diagnostics and vaccine development.
Recombinant antibodies, created with specific amino acid sequences, provide a boon in healthcare. Their targeted binding abilities are critical in diagnostics and therapeutics, as they can, for instance, target specific cancer cells and distinguishing them from healthy tissue.
evitria is specialized in the transient expression of recombinant antibodies. Tailored for individual requirements, we produce rAbs in Chinese hamster ovary cells, providing partners around the globe with high-quality immunoglobulins for diagnostic and therapeutic purposes.
Having antibodies in your blood generally indicates a prior exposure to a specific antigen, which could be a result of a past infection, vaccination, or immune response to a foreign substance. Antibodies are produced by the immune system as a response to these encounters. They play a critical role in recognizing and neutralizing the antigen upon reexposure, contributing to immune memory and protection against future infections.
One example of an antibody is IgG (Immunoglobulin G). IgG is the most abundant antibody class in the bloodstream and plays a crucial role in providing long-term immunity against various infections. It recognizes and binds to a wide range of pathogens, marking them for destruction by immune cells.
There are several types of antibodies, each with specific functions within the immune system. The primary classes of antibodies, or immunoglobulins, include: