Different types of monoclonal antibodies (mAbs) have been developed since the advent of the “golden age of molecular biology” and recombinant protein expression. But how are mAbs actually categorized into types?
A classification can be made according to the method of production, the type of diseases where they are used or the type of modification. However, the naming of the antibody often already reveals something about the type of monoclonal antibody.
Monoclonal antibodies (mAbs) can be produced using different methods, resulting in four main types based on their origin. Here are the four types of monoclonal antibodies and their respective naming:
These antibodies are fully derived from mice. The variable regions of both the heavy and light chains are of mouse origin. Murine mAbs are named using the prefix “o-” or “-omab” at the end of the generic name. For example, “rituximab” is a chimeric antibody that contains murine variable regions and is used to treat certain types of lymphoma and autoimmune diseases.
Subscribe to our Newsletter
Get all the latest updates, and learn about our advancements in antibody production.
Subscribe now
Chimeric antibodies are composed of human constant regions and mouse variable regions. The constant regions determine the antibody’s effector functions, while the variable regions provide antigen specificity. Chimeric mAbs are named using the suffix “-ximab” at the end of the generic name. For example, “infliximab” is a chimeric antibody that targets tumor necrosis factor-alpha (TNF-α) and is used to treat inflammatory diseases such as rheumatoid arthritis.
Humanized antibodies have a majority of human sequence, with only a small portion of mouse sequence in the complementarity-determining regions (CDRs). Humanizing reduces the immunogenicity associated with murine mAbs. Humanized mAbs are named using the suffix “-zumab” at the end of the generic name. For example, “trastuzumab” is a humanized antibody used in the treatment of HER2-positive breast cancer.
Fully human antibodies are entirely derived from human sequences, both in the constant and variable regions. They are designed to minimize immunogenicity and maximize compatibility with the human immune system. Fully human mAbs are named using the suffix “-umab” at the end of the generic name. For example, “adalimumab” is a fully human antibody that targets TNF-α and is used to treat various autoimmune diseases such as rheumatoid arthritis and psoriasis.
These four types of monoclonal antibodies differ in their composition, origin, and potential immunogenicity. The naming conventions help to identify and classify them based on their structure and development process, providing important information for clinicians and researchers working with these therapeutic agents. How exactly the naming is done will be explained in more detail in the next chapter.
Interestingly, researchers and companies that produce monoclonal antibodies are not allowed to freely name their discoveries. The International Nonproprietary Name (INN) as defined by the World Health Organization for monoclonal antibody drugs is codified and the naming follows a regulated scheme. This set of rules defines the INN as consisting of a prefix, infixes and a suffix. While the prefix is variable and can be chosen by the developer, the infixes and suffix have to be selected from a list of defined syllables which specify the antibodies’ target, source and the immunoglobulin structure, with a concluding “-mab”. An example would be trastuzumab:
Tras-: prefix, freely chosen
-tu-: infix, target: tumor
-zu-: infix, structure: humanized constant region
-mab: suffix, monoclonal antibody
For mAbs that are developed from 2022 onward, the convention was adapted to a single infix determining the target and a suffix for specifying the structure.
Prefixes may be chosen freely at the developer’s discretion. Usually, the prefix is chosen to have a pleasant ring to it and should result in a unique-sounding, easy to pronounce name. Being bound to a rigid nomenclature, developers will take a lot of consideration to come up with a great prefix, as it influences the marketability of their product.
The historic two infixes define the target and constant region platform, onto which the antigen binding region is grafted.
Examples for the first infix are -ci- for cardiovascular, -tu- for tumor, -vi- viral, -li- immune system.
The second infix defining the source might be: -i- for primate, -o- for murine, or -xi- for chimeric constant region (>50% human), -zu- for humanized constant region (>90% human) or -xizu- for chimeric/humanized hybrid antibodies. Historically, mAb names end with -mab.
To give an example, the name “-viximab” hints at an antibody that targets a viral antigen and is grafted onto a chimeric Ig-platform.
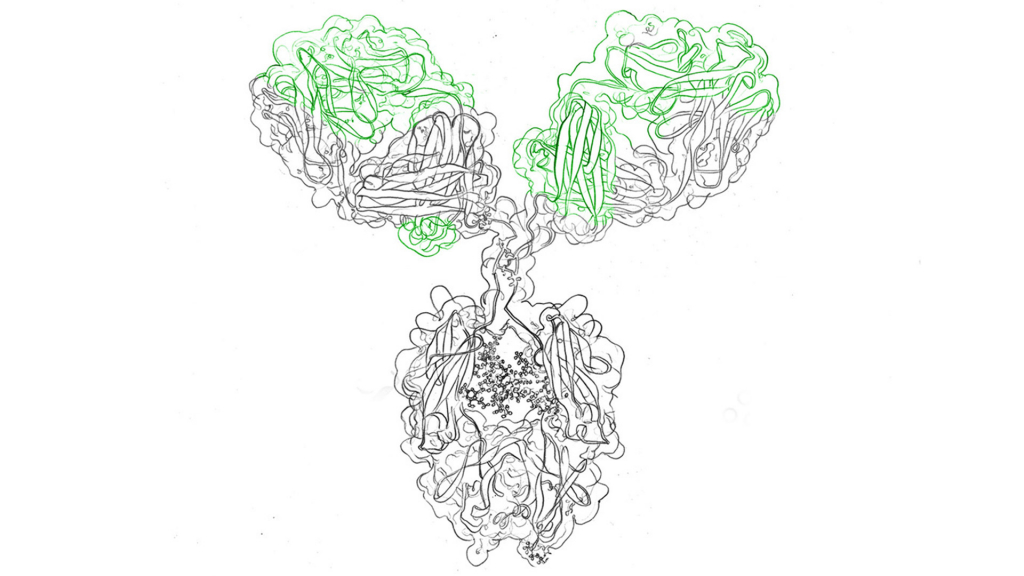
With the latest revision of the nomenclature for mAbs, the suffixes give information about the immunoglobulin structure:
To give timely examples for blockbusters and antibody treatments for SARS-COV-2 (“coronavirus”):
Additionally, a second word may be added to the name to denote further modifications to the mAb:
There are three types of monoclonal antibodies commonly used to treat cancer:
These monoclonal antibodies are unmodified and deliver their therapeutic effects through direct binding to specific targets on cancer cells. They can activate immune responses or inhibit signaling pathways. Unlike conjugated antibodies, naked antibodies do not have additional attached agents. They are widely used in cancer immunotherapy.
Conjugated antibodies, also known as antibody drug conjugates (ADCs), are a type of monoclonal antibody that is linked or conjugated to other therapeutic agents, such as chemotherapy drugs or radioactive isotopes. This conjugation allows for targeted delivery of the attached therapeutic agent directly to cancer cells. The antibody component of the ADC recognizes and binds to specific antigens on cancer cells, ensuring selective delivery of the cytotoxic payload. This targeted approach improves the efficacy of the therapy while minimizing damage to healthy tissues. Antibody drug conjugates have emerged as a promising strategy in cancer treatment, offering a combination of antibody specificity and the potent cytotoxic effects of the attached agent. By leveraging the antibody’s ability to recognize cancer cells, ADCs enhance the delivery and localized release of the therapeutic payload, leading to more effective tumor killing.
Bispecific monoclonal antibodies are engineered to simultaneously bind to two different targets. This dual binding capability allows them to bring cancer cells and immune cells together, enhancing the immune response against cancer. Bispecific antibodies can redirect immune cells to attack cancer cells directly, offering a unique approach to cancer treatment.
These different types of monoclonal antibodies used in cancer therapy provide diverse mechanisms of action and therapeutic strategies. Naked antibodies rely on their binding specificity, conjugated antibodies combine targeted delivery with cytotoxic agents, and bispecific antibodies engage the immune system for enhanced tumor targeting and destruction.
Monoclonal antibodies (mAbs) have shown efficacy in treating a wide range of diseases. Some of the diseases that can be treated with monoclonal antibodies include:
Monoclonal antibodies have revolutionized cancer treatment. They can target specific proteins or receptors on cancer cells, inhibiting their growth, promoting immune responses, or delivering cytotoxic agents directly to the tumor.
Monoclonal antibodies are used to treat various autoimmune disorders such as rheumatoid arthritis, psoriasis, lupus, and inflammatory bowel diseases. They can modulate the immune response and reduce inflammation associated with these conditions.
Monoclonal antibodies have been developed to combat certain viral infections, including respiratory syncytial virus (RSV), HIV, and Ebola. They can neutralize viruses, prevent their entry into host cells, or facilitate their clearance by the immune system.
Monoclonal antibodies are being investigated for the treatment of neurological conditions like Alzheimer’s disease, multiple sclerosis, and migraine. They target specific molecules involved in the pathology of these disorders to potentially slow disease progression or alleviate symptoms.
Monoclonal antibodies are used to manage allergic diseases, such as asthma and allergic rhinitis. They target molecules involved in the allergic response, reducing inflammation and alleviating symptoms.
Monoclonal antibodies have been developed for the treatment of ophthalmic conditions like age-related macular degeneration and diabetic retinopathy. They can inhibit abnormal blood vessel growth or inflammation in the eye.
Monoclonal antibodies are employed to prevent organ transplant rejection by targeting immune cells or molecules involved in the rejection process.
These are just a few examples of the broad spectrum of diseases that can be treated with monoclonal antibodies. The versatility and specificity of mAbs make them valuable therapeutic options across different medical fields.
While all drugs on the market must undergo in-depth investigations with clinical trials to assess their reward-risk-ratio before gaining approval by the US Food and Drug Administration (FDA), highly rewarding mAbs used in oncology also potentially cause serious side effects.
Monoclonal antibody therapies are usually administered by specialized health care providers and antibody infusion centers. They require either subcutaneous injection or more often intravenous infusion, thus any side effects may appear in a fulminant fashion: rashes, changes in blood pressure, shortness of breath, heart rate increases. In very rare cases, anaphylaxis, cytokine release syndrome for immunotherapies2, or tumor lysis syndrome might occur, which require immediate intervention.
Moreover, monoclonal antibodies can be classified by ways of their production:
Read more: How are monoclonal antibodies produced in the lab?
Recombinant antibodies are a class of their own due to their unique development. Since recombinant DNA technology is used to design the sequence, full control of the antibody structure is possible. Even single mutations can be introduced to optimize nearly every property.
For many, the next generation of mAbs is a beacon of hope to touch even more patients’ lives and make mAb treatments safer and more effective.
The most common monoclonal antibodies include trastuzumab, rituximab, and adalimumab, used in the treatment of various cancers, autoimmune diseases, and inflammatory conditions, respectively.
Current monoclonal antibodies used for COVID-19 include bamlanivimab, casirivimab/imdevimab, and sotrovimab. They are authorized for the treatment of mild to moderate COVID-19 in certain patients.
Examples of monoclonal antibodies include pembrolizumab, infliximab, and bevacizumab, used in the treatment of cancer, autoimmune diseases, and eye conditions, respectively. Many other examples exist for different therapeutic indications.