Recombinant antibodies are antibodies that have been genetically engineered to overcome the limitations of other methods of antibody expression, such as genetic drift. Apart from high affinity, improved levels of sensitivity and confirmed specificity, they offer a higher consistency between batches than traditional monoclonal antibodies as well as continuous large-scale supply and high-throughput.
Monoclonal antibodies are increasingly popular and important tools in the fields of biochemistry and medicine. They can be produced using different technologies and while there are many kinds of different monoclonal antibodies – be it a primary or secondary antibody – each type binds to one antigen only. This is why antibody libraries can be searched for existing antibodies with the right characteristics for specific uses.
Monoclonal antibodies are made by cloning a white blood cell, which is always unique. All subsequent antibodies derived this way trace back to this unique parent cell, while polyclonal antibodies are usually derived from several different plasma cell lineages.
As the name suggests – and opposed to polyclonal antibodies –, monoclonal antibodies have a so-called monovalent affinity. In other words, they are only binding to the same epitope.
Subscribe to our Newsletter
Get all the latest updates, and learn about our advancements in antibody production.
Subscribe now
Read more: What are polyclonal antibodies? | Monoclonal versus polyclonal antibodies
There are different methods for large-scale monoclonal antibody production and validation, but in order to achieve adequate amounts of functional antibodies, the production process usually starts with the generation of mAb-producing cells by fusing myeloma cells with desired antibody-producing B cells that are typically sourced from mammalian cell lines.
Monoclonal antibodies can be produced using hybridoma cell cultures and harvested as hybridoma tissue culture supernatants. Hybridoma technology includes the generation of a cell line to produce a single antibody and starts by injecting a mouse with an antigen to provoke an immune response. Hybridoma cell lines can be maintained and harvested indefinitely, which is a major benefit. However, the involvement of animals during immunization or the risk of mutations are seen as disadvantages of mAbs. In addition, hybridoma expression is a laborious process and results in long lead times – not ideal when time is of the essence.
Phage display technology works by genetically engineering bacteriophages to display antibodies on their surface. This allows the binding of bacteriophages with desired antibodies onto immobilized antigens. The high affinity binders are then used to infect bacteria and collect their DNA for sequencing. This process isn’t only much faster than hybridoma production, it also doesn’t require any animal immunization.
Finally, single B cell technologies describe the screening and sorting of ex vivo cultures of single B cells that have been taken from blood samples of immunized animals or humans. This approach involves the extraction of genetic information as well as amplification, sequencing and cloning into expression vectors for mAb production and doesn’t require any hybridoma cell lines, which considerably speeds up the production process.
Read more: What is an antibody sequencing service? | How are monoclonal antibodies produced in the lab?
Thanks to their specificity, monoclonal antibodies have become an important pharmaceutical and medical tool, with a wide range of applications, such as:
Therapeutic full-length antibodies, which require complex post-translational modifications, are predominantly produced using stable mammalian expression systems whereas antibodies intended for diagnostics and research can be produced using bacterial and yeast expression systems.
While monoclonal antibodies only bind to one epitope, it is possible to enhance them by means of artificial engineering. Apart from these capabilities, mAbs stand out with advantages such as
Recombinant antibody technology is a rapidly evolving field that enables the targeted improvement of antibody properties – including antigen-binding site affinity, reactivity and specificity, but also isotype conversion – by means of genetic engineering. The functional expression of antibody fragments in E. coli has formed the basis for antibody library generation and selection, a powerful method to produce various types of antibodies – IgG antibodies and its subtypes (like IgG1) being the most important one – used for medical therapies.
One of the most common and important recombinant antibody formats for the life-science industry is the single-chain variable antibody fragment (scFv). As ScFv fragments retain the binding specificity of the parent antibody, they offer several advantages compared to full-length mAbs. But what is a recombinant antibody?
Recombinant antibodies are monoclonal antibodies that are generated using recombinant DNA – synthetic genes and antibody fragments – rather than hybridomas. Unlike conventional monoclonal antibody production, which requires immunizing animals with immunogens to provoke immune responses, the host cells for recombinant antibodies are generated in an animal-free process.
What is a recombinant monoclonal antibody?
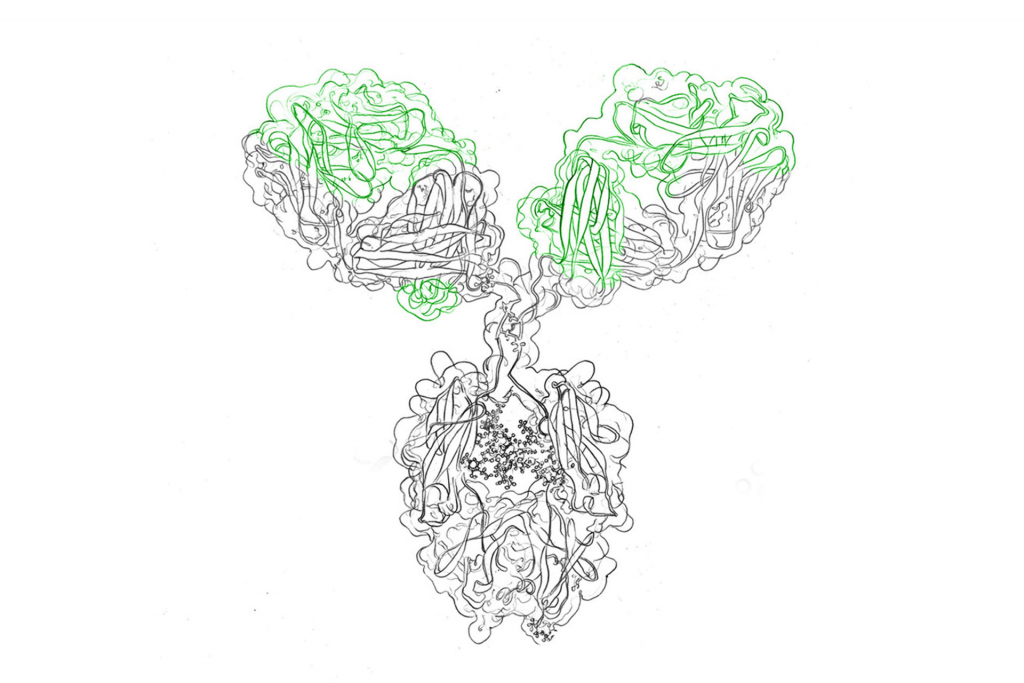
Recombinant antibody production allows for the cloning from any species of antibody-producing animal, provided there are appropriate oligonucleotide primers or hybridization probes available. The ability to manipulate the antibody genes also allows for the generation of new antibodies and antibody fragments (Fab fragments and scFv) in vitro. Display libraries, comprising heavy chain as well as light chain diversities, commonly expressed in phage or yeast, can then be analyzed to select a specific antibody based on desirable characteristics arising from such changes in antibody sequence.
Recombinant antibodies provide excellent sensitivity, making them suitable for reagents needed to analyze low-abundance targets or detect multiple post-translational modifications at once.
Like monoclonal antibodies, there is a number of applications for recombinant antibodies, most notably
According to experts, recombinant monoclonal antibody production has revolutionized the generation of immunoglobulins.1
Recombinant antibodies offer a number of advantages, most notably their high quality and their reproducibility. They are easier and faster to produce than monoclonal antibodies, while at the same time offering the added benefit of being more consistent and scalable.
In addition, they can be engineered to improve specificity. As an example, variable region engineering of IGG antibodies improves antigen binding properties, pharmacokinetics, pharmaceutical properties and immunogenicity. They can be conjugated with other molecules such as biotin, enzymes (HRP, AP, etc.), and fluorochromes (FITC, PE, APC, etc.) for research purposes and conjugated with toxins and drugs for therapeutic use.
Recombinant antibodies generally come with all advantages of monoclonal antibodies, such as high selectivity and homogeneity, suitable for diagnostic and therapeutic applications.
The use of in vitro methods over laboratory animals allows full control of the process and fine-tuning of the desired parameters. So, while abolishing the use of animals as in the production of traditional monoclonal and polyclonal antibodies, the production of recombinant antibodies also pushes advances in the fields of molecular biology and recombinant DNA technology, which is considered one of the most effective methods for enhanced production of proteins, peptides or enzymes.