Recombinant antibody production
We are a leading service provider for recombinant antibody production. Get in contact!
Recombinant protein production has become a cornerstone of biotechnology and will stay a major growth driver for the biotech market in the present decade1. One major reason is the growing demand for recombinant antibodies as innovative biopharmaceuticals, since they are expected to demonstrate higher therapeutic efficacies along with reduced side-effects compared to traditional therapeutics.
With this article, we want to take our readers on a tour through the basics of recombinant protein productions and highlight some noteworthy details of the involved processes.
Recombinant proteins are proteins that are made in the laboratory by living cells that follow the instructions encoded in recombinant DNA sequences. Recombinant proteins might be completely artificial by using DNAs that have no counterpart in natural sources, although they might be indistinguishable from natural proteins if the sequence is identical to a natural genetic blueprint and gene expression is done in a suitable host organism.2
Protein production is the process of creating protein matter from simple substrates, usually by employing biotechnological means, i. e. suitable hosts. The host cells are mostly prokaryotic single cellular organisms such as bacteria (E. coli), yeasts or eukaryotic mammalian cell lines which are genetically engineered using expression vectors to produce the protein of interest in high yields. The target proteins are then removed from the culture medium and purified into biopharmaceuticals or reagents.3
Subscribe to our Newsletter
Get all the latest updates, and learn about our advancements in antibody production.
Subscribe now
One key reason is the improvement in the production of recombinant proteins, leading to better availability and continually increasing demand for specific recombinant proteins as diagnostic reagents (e.g. fluorescence probes and ELISA assays) and of course highly promising pharmaceuticals such as recombinant antibody therapeutics or vaccines.
Worth of note is the development of recombinant protein polymers for sustainable, animal-free, not mineral oil derived and microplastics-free personal care products4.
Now we want to take a closer look into recombinant protein production and subsequent downstream processes that involve recombinant protein purification:
At the beginning of recombinant protein production stands the preparation of the gene of interest from the genome of the species that produces a related peptide or protein. Since the expression host would not naturally express these proteins, they’re called heterologous proteins.
Modern recombinant methods allow the joining of DNA molecules stemming from different species (resulting in fusion proteins), using RNA from in vivo samples by reverse transcription and PCR to generate cDNA.
The gene undergoes several rounds of optimization and is then equipped with the necessary regulatory elements (e.g. promoters that can be activated by the addition of IPTG in Escherichia Coli) or labeling with affinity tags to allow for efficient protein overexpression and purification. After embedding the sequence into plasmids and subsequent amplification, the constructs are inserted into bacterial cells or mammalian cells.
The host cells are then kept in flasks for small scales such as high-throughput experiments for screening for variants or mutations, or alternatively in bioreactors for large-scale manufacture. The cells are grown under tightly controlled culture conditions to facilitate optimal metabolic states for maximal cell growth and protein synthesis during the fermentation phase. When the cell culture reaches optimal cell densities, the proteins will be harvested.
While some soluble proteins undergo secretion to the culture media, aggregation occurs at high protein yield and many proteins, especially membrane proteins, are concentrated in inclusion bodies. Therefore, cells are preferably lysed by chemical means (enzymes or detergents such as SDS) to gain access to the target proteins. Since biomacromolecules are susceptible to degradation by shear forces, physical lysis methods are to be used cautiously.
At this stage, the crude lysate contains all contents of the host cells along with the recombinant protein of interest. Problematic is the presence of proteases which begin to degrade the target protein immediately after lysis. Therefore, the crude solution is cooled and quickly subjected to a cascade of separation and purification steps to remove unwanted substances and enrich the target protein in high-quality. If affinity tags were used in the design of the recombinant protein, the target protein can be fished from the preprocessed solution by affinity chromatography.
Read more: Recombinant antibody purification
Finally, the pure recombinant protein is put into storage in the frozen state.
A small sample of the product is then put through all the analytical tools of proteomics to obtain a complete characterization of its chemical identity, amino acid residue sequence, purity, impurity profiles, absence of microbial contaminants and for antibodies or receptors as well as perhaps their specificity towards antigens or ligands.
A successful recombinant protein production campaign depends on the correct choice of expression hosts. While the highest quality of compatibility to the human physiology is generally achieved by using mammalian cells, this is not always necessary.
Now, we want to highlight several popular expression hosts.
Further readings: Recombinant antibody expression
The method of recombinant protein production was first developed using bacteria (E. coli strains) as host organisms. Due to their simple cell biology, scientists first understood connections between DNA and RNA as templates for polymerases. The ribosome reads the codons on mRNA strands and assembles amino acids to produce peptides and proteins.
While bacteria are very cost-efficient due to their ease in handling and high recombinant protein yields, for complex proteins stemming from eukaryotic sources, they are often non-functional due to incorrect folding or absent post-translational modifications. Such proteins should be expressed in eukaryotic host organisms.
The use of insect cells for recombinant protein production was a relatively early addition to the toolbox and offers advantageous post-translational modifications (similar to mammalian systems) at high expression levels.
Popular protocols employ recombinant viral particles that can be loaded with the genetic material coding for the protein of interest, which then infect the cultured insect cells and reprogram them to express the desired protein in very high yields.
Although recombinant protein expression in mammalian cells is accompanied by challenges in handling the cell cultures, the advantages are not just incremental, but of categorical nature.
The technique of CHO cell culture makes use of evolutionary highly advanced mammalian cell biology that yields protein with intact post-translational modifications such as facilitating folding into the correct 3D structure via chaperones and disulfide bond formation and glycosylation patterns via dedicated enzymes. This is a huge advantage for therapeutic proteins, due to lower chances for side effects.
We are a leading service provider for recombinant antibody production. Get in contact!
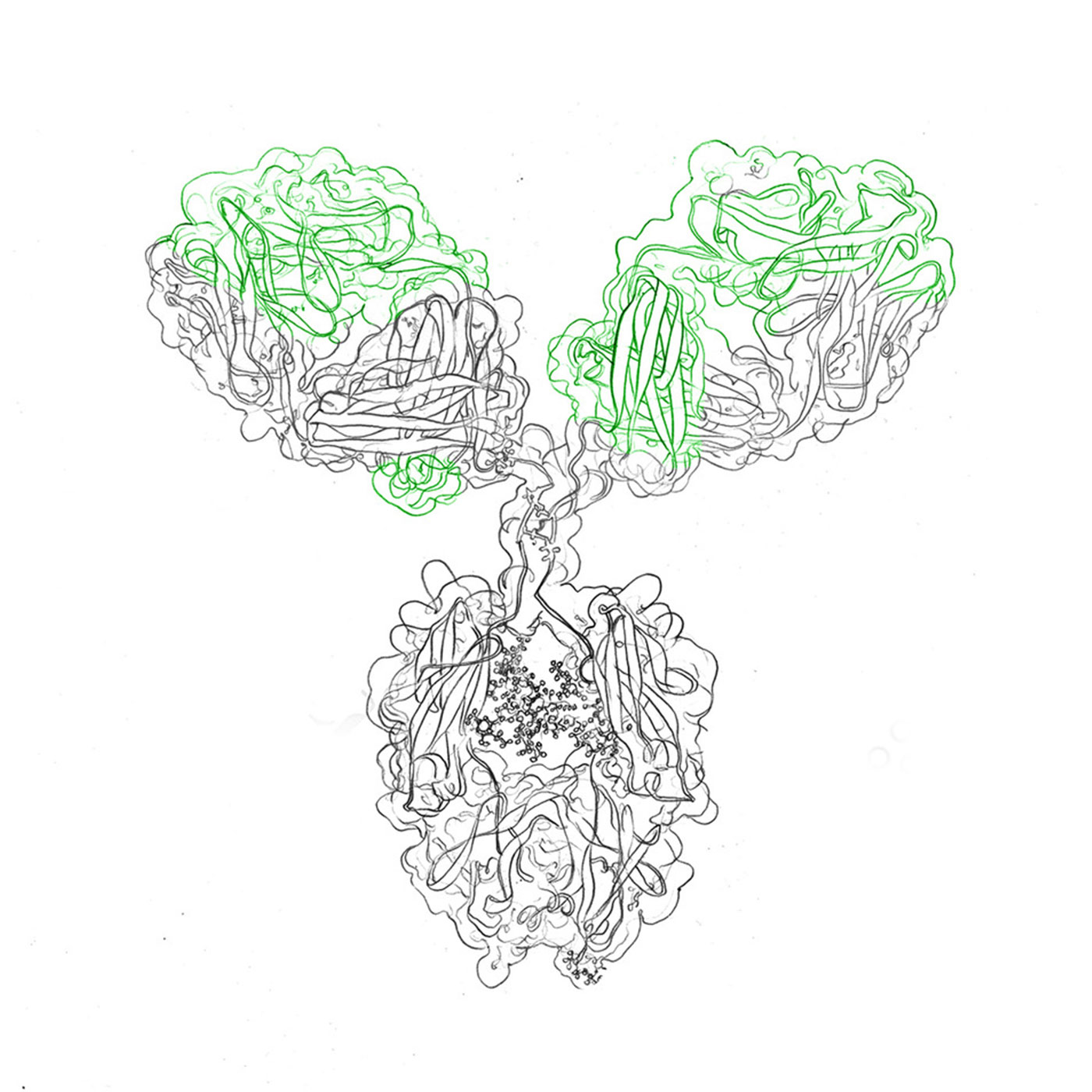
Alternatively, cell-free methods for recombinant protein production are available. In vitro translation (IVT) relies on translation-competent cell extracts that contain all cell constituents necessary for protein synthesis, thus avoiding the need for actual cell culture. This method is not suitable for scale up.
In this section, we want to give our readers a succinct overview over the pros and cons of different protein expression systems and how they compare to each other:
Expression system Pros Cons Notes Bacteria easy handling
good yields
cost-efficientnot suitable for complex proteins
no post-translational modificationspopular for general applications, diagnostics, etc. Yeast easy handling
post-translational modificationsok yields
refolding often requiredpopular for simpler proteins that still require post-translational modifications Insect folding often correct
post-translational modifications often okcell culture not easy
medium yieldsimmunogenicity and functionally more similar to mammalian proteins than yeast-expressed proteins Mammalian / CHO folding correct
post-translational modifications correctcell culture difficult
slow cell growthhighest quality in terms of immunogenicity, essentially human-like
ideal for pharmaceuticals due to great side effect profilescell free no cell culture required doesn’t scale well suitable for laboratory work
Recombinant protein expression has become a multi-billion dollar market and novel ways to improve the process key performance indicators are major achievements, economically speaking.
Synthetic biology approaches are applied on expression hosts to alleviate the metabolic impact of reprogramming them to express unnaturally large amounts of proteins5. These advancements aim to make recombinant protein expression more efficient.
Another example targets inclusion bodies (IBs), where cells tend to deposit protein of low solubility. Significant amounts of protein are lost in IBs. Researchers developed methods employing freeze-thaw cycles to increase recovery rates from inclusion bodies.6
Recombinant protein expression and purification can be considered the pinnacle of recombinant protein manufacture and companies offering recombinant antibody expression service unify profound knowledge of eukaryotic cell biology, processes involving complex glycoproteins and have large experience with mammalian cell culture.
At evitria we offer recombinant antibodies produced in CHO cells using a transient expression protocol. This flexible expression platform allows the fine-tuning of post-translational modifications such as glycosylation patterns.
The product portfolio also contains non-antibody protein products such as viral proteins, soluble extracellular domains, cytokines, and many more.
Recombinant proteins are produced by inserting a specific gene into a host organism, typically bacteria or yeast. The host organism then uses this gene to produce the desired protein, enabling large-scale production for various applications.
Recombinant protein production is a process where scientists use genetic engineering techniques to insert a specific gene into a host organism, such as bacteria or yeast. This host organism then produces the desired protein based on the inserted gene’s instructions. This method allows for the mass production of proteins for various purposes, including medical treatments, research, and industrial applications.
Recombinant DNA is a DNA strand that has been created by using recombinant technology, i. e. molecular cloning technology using restriction enzymes to modify or combine gene fragments into artificial genetic material. Recombinant DNA is usually equipped with promoter regions and regulatory elements to optimize transcription, translation and proper protein folding of the target compound.
Recombinant protein production is important because it allows for the large-scale production of specific proteins that are crucial for various applications. This includes producing therapeutic proteins for medical treatments, enzymes for industrial processes, and research tools for scientific investigations. It offers a controlled and efficient way to obtain proteins that may be challenging to isolate or produce through traditional methods.
Recombinant protein production services are offered by various companies, research institutions, and biotechnology firms specializing in bioprocessing and genetic engineering. Organizations, such as Evitria, have the expertise and infrastructure to produce custom proteins for research, therapeutic, or industrial purposes.
The costs for recombinant protein production can vary widely depending on several factors, including the complexity of the protein, the production scale, and the specific service provider or facility used.