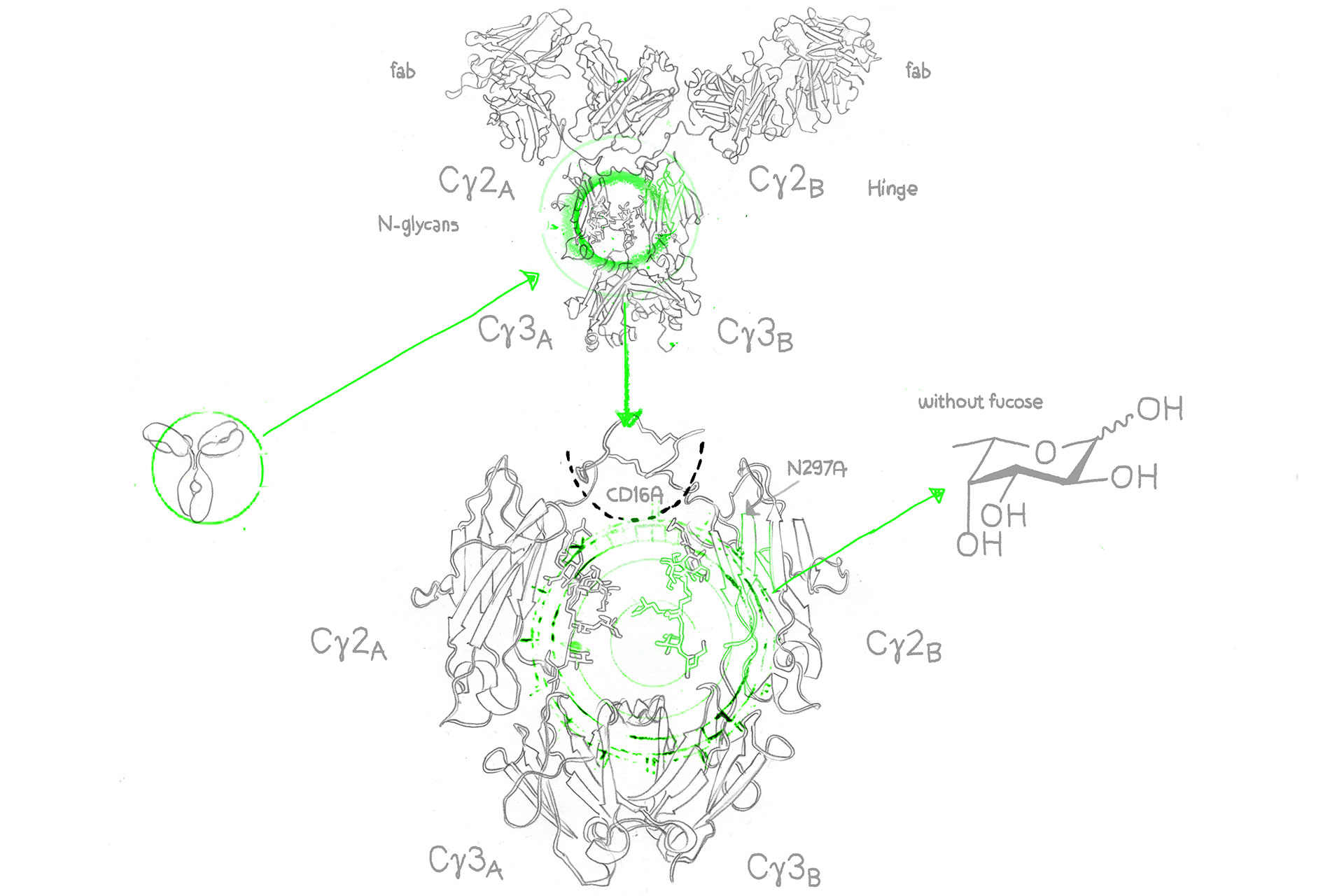
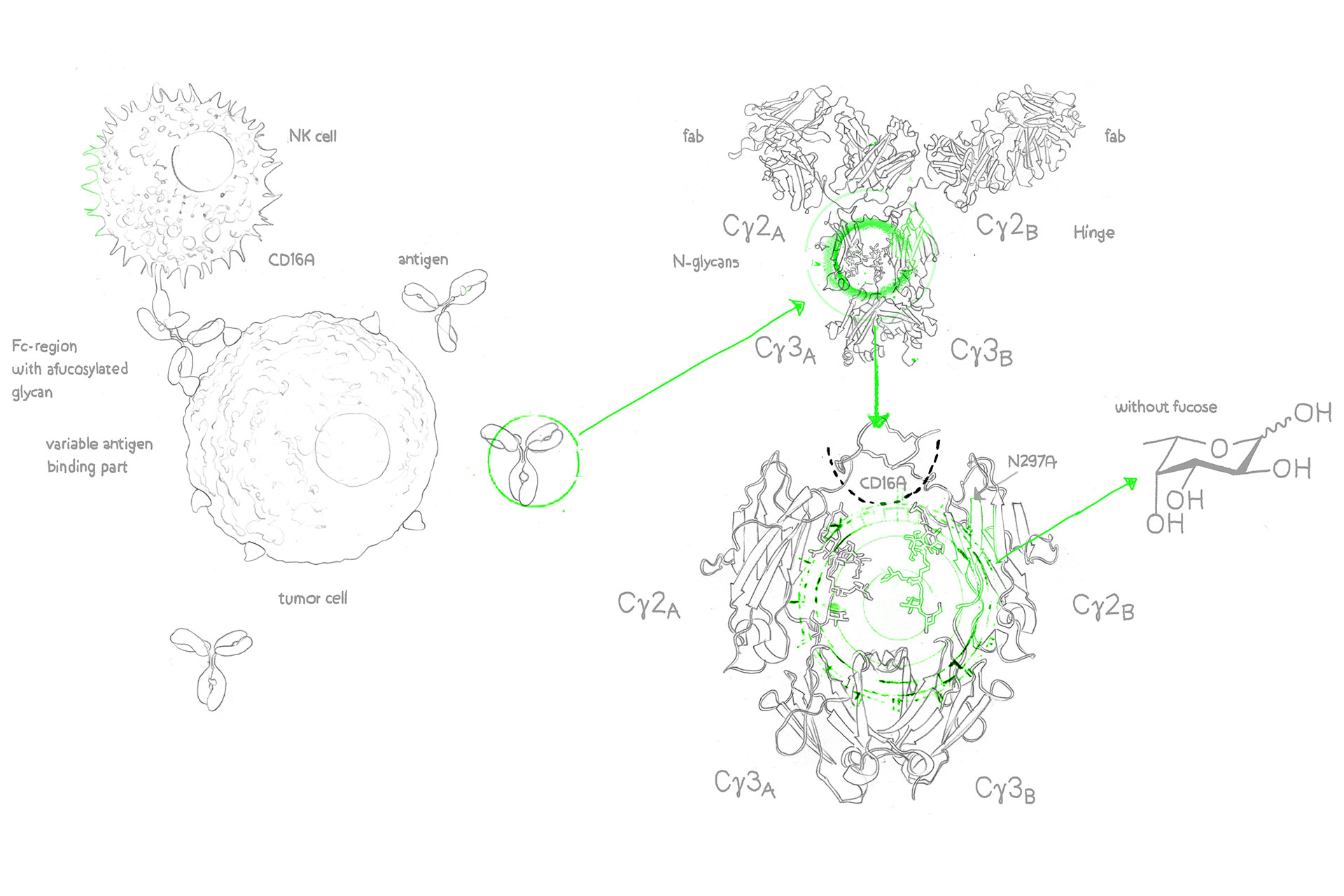
Therapeutic antibodies with low fucose content demonstrate higher ADCC (Antibody-Dependent Cellular Cytotoxicity), i.e. elevated killing activity against tumorigenic and infected cells.
Artificially decreasing fucose content via afucosylation can enhance ADCC and thereby increase therapeutic effect, significantly reduce dosage requirements, and eventually result in fewer side effects.
evitria holds an exclusive licensing partnership to offer afucosylation via ProBioGen’s GlymaxX® technology for your transient antibody expression needs.
GlymaxX® antibodies purified
GlymaxX® projects completed
With the GlymaxX® platform, evitria can transiently express both native and afucosylated variants using the same CHO cells, the same DNA, and without any amino acid sequence modifications. In other words, we can provide the perfect negative control.
Whereas alternative afucosylation approaches rely on Fc mutations that introduce risk of immunogenicity, evitria’s use of GlymaxX® produces the same yields and stability as native antibodies without the danger of potential immune response.
As a service provider for recombinant antibody production we focus on this service only. This enables us to offer highest quality standards and fast turnaround times for afucosylated antibody expression.
From sequence to antibody in 5 weeks
Project start
0-1 business days
Pilot study
(from electronic sequence to quantified pilot supernatant)
15-20 business days
Larger-scale expression
5-10 business days
Purification
2-3 business days

Final analytics
2-3 business days
Shipment
1-2 business days
How to order with evitria
You tell us what you need and when
Optional: We discuss your project and how we might be able to assist in a personal conversation
You receive our proposal within one working day
You review and approve our proposal
Your project begins within one working day
When therapeutic antibodies are required for the next step in scientific research, recombinant antibody production must not be a bottleneck. On the contrary: Both quality standards and time schedules need to be granted by the antibody provider.
We at evitria are not only specialized in afucosylated antibody expression, but also in
Additionally, we provide a careful sequence and project review in order to deliver quality products for your research – from sequence to antibody in 5 weeks.
Time is key – we therefore complete pilot studies within 15 to 20 business days!