Custom recombinant antibody production
We are a leading service provider for custom recombinant antibody production. Get in contact!
Antibody engineering has emerged as a transformative force in biotechnology and immunology, reshaping the landscape of diagnostics and therapeutics. This dynamic field in antibody production harnesses the power of antibodies – versatile proteins that naturally defend our bodies – and enhances their capabilities through precise manipulation.
In this blog post, we will explore the various expression systems and fundamental principles that underpin this science. We delve into the opportunities it presents, from the creation of high-affinity antibodies to the development of innovative formats like bispecific antibodies and antibody-drug conjugates. Simultaneously, we confront the challenges, considering factors such as immunogenicity, toxicity, and the careful navigation of clinical trials and regulatory approval, that come with antibody engineering.
The canvas of antibody engineering stretches far and wide, coloring the landscape of healthcare with transformative applications. From antibody therapeutics that target diseases with precision, to next-generation formats that challenge conventions, the potential is boundless.
Antibodies become therapeutic arrows, precisely aimed at disease targets, minimizing collateral damage to healthy cells. These antibodies empower the immune system, bolstering its natural defenses and ushering in a new era of immunotherapy.
Subscribe to our Newsletter
Get all the latest updates, and learn about our advancements in antibody production.
Subscribe now
Next-generation formats, including nanobodies and domain antibodies, offer compact yet powerful tools. These innovations navigate intricate cellular landscapes, addressing challenges once thought insurmountable.
Beyond therapies, antibodies find their place in diagnostics, serving as reagents to detect disease markers with remarkable sensitivity. Their precision enables early intervention and personalized treatment approaches.
Moreover, antibodies’ modular nature drives innovation. Their binding domains can be engineered to target specific residues, offering endless possibilities for tailored therapies and treatments.
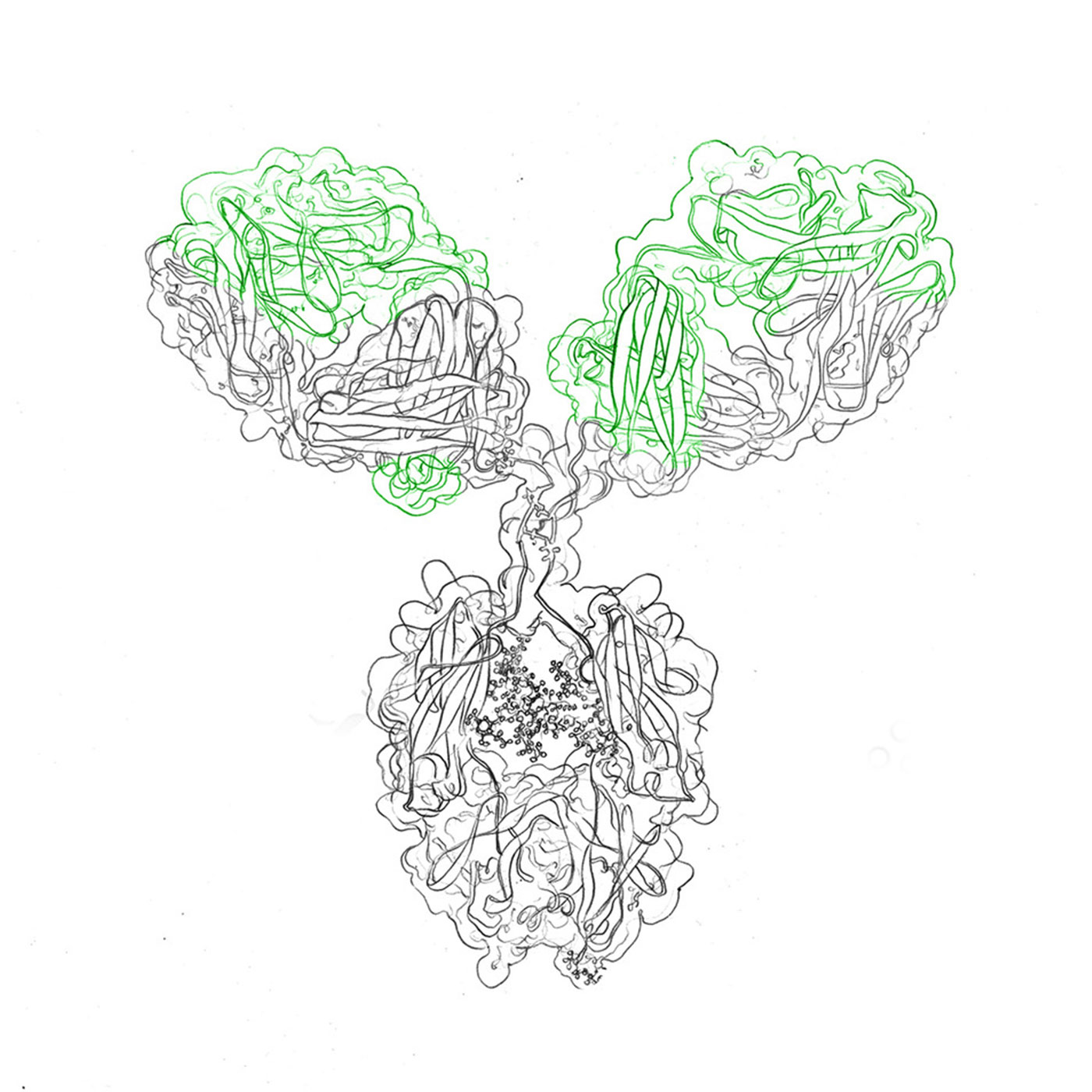
In antibody engineering, fundamental principles guide the design and function of these remarkable molecules. From the architecture of immunoglobulins to their role in receptor interactions and cell surface recognition, understanding these principles is essential for harnessing engineered antibodies’ potential.
Antibody structure and function: Antibodies, like immunoglobulin G (IgG), consist of heavy and light chains, forming a Y-shaped configuration with variable and constant regions. These regions grant antibodies their specificity and binding affinity.
Receptor interactions: Antibodies bridge antigens and immune cells through interactions with cell surface receptors. This triggers immune responses like phagocytosis, cytokine release, and antibody-dependent cellular cytotoxicity (ADCC), enabling tailored immunotherapies.
Cell surface recognition: Antibodies recognize specific antigens on cell surfaces, initiating immune surveillance and targeted interventions. Antibodies’ affinity for cell surface antigens determines their efficacy.
scFv antibodies: Single-chain fragment variable (scFv) antibodies retain antigen-binding capacity while comprising only variable domains of heavy and light chains. Their modular nature facilitates customization.
The core of antibody engineering encompasses sophisticated techniques enabling the manipulation and optimization of antibodies to meet specific needs. These methods, from phage display and hybridoma technology to antibody production in animals, single B cell technology, recombinant DNA approaches and advanced display technologies, form the foundation of innovation in this dynamic field.1
Techniques in antibody engineering serve various applications, from antibody-drug conjugates (ADCs) to next-gen formats like the production of bispecific antibodies. These methods underpin innovations driving therapeutic, diagnostic, and biotechnological progress.
The pivotal choice of an expression system shapes the trajectory of antibody engineering, steering the course of outcomes. In this dynamic arena, the terrain spans both in vivo and in vitro domains, each offering unique avenues.
In vivo expression: Harnessing living organisms for antibody generation bestows native post-translational modifications and folding. Yet, challenges like ligand interference and variable half-life necessitate judicious consideration.
Read more: In vivo antibodies
In vitro expression: Controlled settings of cell-free systems, microbial hosts or mammalian cell lines simplify production, sidestepping in vivo complexities. Consistent yields, glycosylation patterns, and antibody functionalities often favor this route.
Read more: In vitro antibody production
Factors like variable regions, peptides, amino acids, and considerations like Fc receptor neonatal (FCRN) interactions, have to be considered, as well as ethical concerns. Navigating these choices also involves understanding the context, such as complementarity-determining region (CDR) diversity, which may impact binding affinity.
Characterizing antibodies and assessing their functionality is crucial for ensuring their efficacy and safety across applications. Various assays illuminate their behavior, providing insights into binding affinity, specificity, and potential variants.
Surface plasmon resonance (SPR) and enzyme-linked immunosorbent assays (ELISA) quantitatively reveal binding kinetics and specificity. Epitope mapping identifies precise binding sites, guiding antibody engineering.
Functional assays gauge immune cell activation. Neutralization assays assess therapeutic antibodies’ inhibition of viral or microbial activities.
Stability, aggregation, and post-translational modifications are examined to ensure long-term effectiveness and minimize risks. Detection and characterization of variants, alongside comparability studies, safeguard product quality and consistency.
In vitro and in vivo assays bridge lab findings with real-world scenarios, confirming antibody behavior. Pharmacokinetic studies track antibody concentration over time, influencing dosing strategies.
Antibody engineering transcends tradition, unlocking a world of potential in therapeutics, diagnostics, and biotechnology. Bispecific antibodies, binding two antigens simultaneously, revolutionize precision targeting. Antibody-drug conjugates (ADCs) merge specificity with cytotoxic agents for pinpoint drug delivery. Compact nanobodies and domain antibodies challenge norms with reduced immunogenicity, expanding therapeutic options.
Customized antibodies in diagnostics enable early disease detection and refined patient stratification. Immune modulation via antibody engineering offers breakthroughs in autoimmune treatments. Synthetic biology ushers in designer antibodies with tailored functionalities.
Ion channels, G protein-coupled receptors (GPCRs), and intracellular proteins emerge as accessible therapeutic targets. These opportunities materialize in clinical trials, transforming potential into impactful treatments.
In antibody engineering, a tapestry of challenges interweaves with innovative strides, demanding vigilant consideration to ensure continued progress. Amidst the promise, potential threats emerge, demanding a meticulous approach.
We are a leading service provider for custom recombinant antibody production. Get in contact!
Stepping from laboratory breakthroughs to clinical applications demands careful orchestration of clinical considerations and regulatory approvals. This intricate journey ensures the transformation of innovative ideas into tangible patient benefits, marked by meticulous planning, stringent evaluations, and regulatory compliance.
Preclinical evaluation, with animal models guiding safety assessments, precedes human trials. Phase I evaluates safety and dosing in a small group, while phase II expands to gauge efficacy and side effects in a broader population. Phase III verifies efficacy and gathers substantial data for regulatory submissions.
Upon successful trials, regulatory submissions undergo thorough scrutiny by agencies like the FDA, ensuring safety, efficacy, and manufacturing integrity.
Post-approval monitoring ensures sustained safety and efficacy insights within real-world contexts.
Maintaining product quality and safety through biomanufacturing compliance is vital. Global harmonization efforts seek consistent standards, and real-world patient experiences contribute to a comprehensive understanding of antibody therapies’ impact.
When facing these challenges, many biopharmaceutical companies and laboratories rely on the services of CDMOs, given their often sole focus on antibody expression services. Hence, CDMOs and CROs can help to mitigate the challenges not only related to regulatory demands, but also in the antibody development process.
Given the enormous complexity of antibody engineering, partners worldwide rely on the recombinant antibody expression services provided by evitria. Focusing on the production of recombinant proteins in CHO cells, we are not only able to deliver high-quality antibodies, but also achieve fast production times to support your projects. In addition, we can help to ensure an optimal outcome by conducting a pilot study prior to recombinant antibody production.
With a broad repertoire of antibody types that can be produced at evitria, we support customers around the globe in their efforts to bring antibody technology and its applications to entirely new levels.