Antibodies are among the most important fighters of the human adaptive immune system. These molecules patrol inside the body, detect invaders, and mark them for destruction by immune cells.
The human body produces natural IgM antibodies against pathogens it hasn’t even been exposed to yet. Circulating B cells upon coming across a pathogen undergo clonal selection and proliferation and the most effective clones proliferate and produce more antibodies to clear the antigen and provide immune protection by deploying various downstream mechanisms. This very fact made antibodies rank among the most crucial tools in medical research and led to numerous novel therapies against hard to treat conditions.
In this text, the reader will learn about the nature of antibodies, what their functions are and how they have been harnessed for research and health care.
Antibodies are large Y-shaped glycoprotein molecules that are composed of two light chains and two heavy chains. The two tips of the “Y” are antigen binding sites that attach to foreign molecules or pathogenic microorganisms (the “antigen”), e.g., the spike protein of the SARS-COV-2 coronavirus.
Subscribe to our Newsletter
Get all the latest updates, and learn about our advancements in antibody production.
Subscribe now
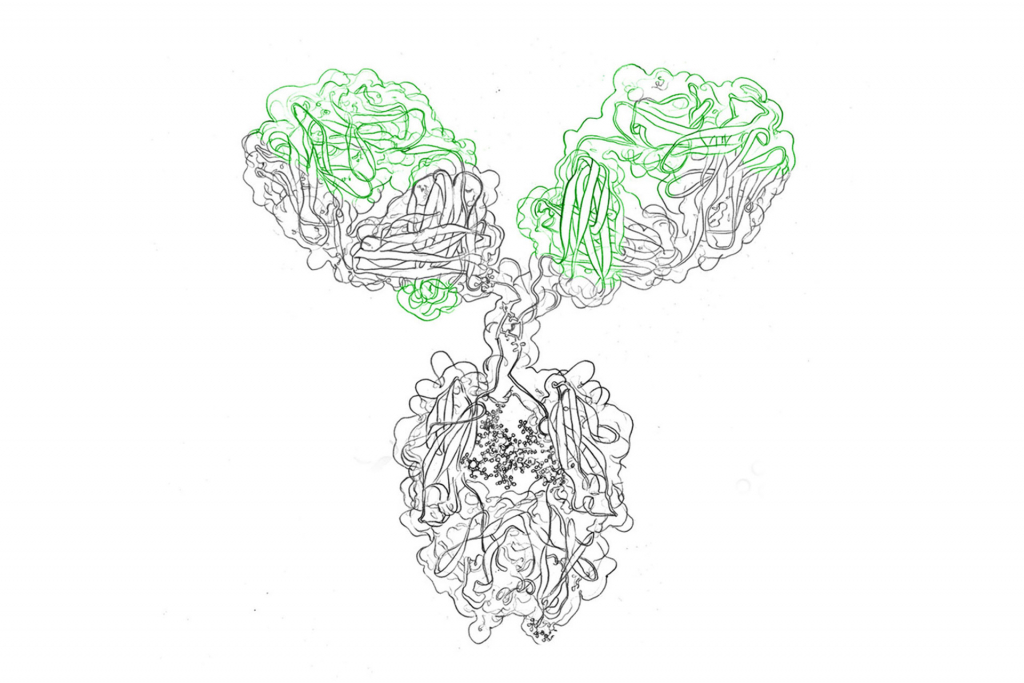
Antibodies are also called immunoglobulins and are classified depending on their type of heavy chain into five variants (isotypes): IgG, IgA, IgD, IgE, IgM. They all have distinct functions in our immune system.
Antibodies are produced by specific white blood cells, the B-cells. Antibodies are located on the cell surface of B-cells and are secreted by plasma cells in large amounts into extracellular fluids e.g., blood plasma and mucosal secretions to fight infectious diseases.
The main role of antibodies is two-fold: binding of antigens and mediation of downstream effector function (biological activities). These functions are performed by different parts of the antibodies’ structure, variable and constant regions. The variable region is concerned with antigen binding and is termed Fab (fragment antigen binding), while the constant region forms the base Fc region (fragment crystallizable ) and is the key player in performing downstream effector functions by communication with other components of the immune system. Immune cells have certain surface receptors (Fc receptors) to bind to antibodies and activate the immune system.
Each type of antibody has a distinct function. For example, the most abundant IgG type Ab conveys long-term immunity against bacteria, viruses, toxins and enhances phagocytosis and ADCC antibody dependent cellular cytotoxicity. IgM is a pentameric molecule and is involved in protection against blood borne antigens and enhances phagocytosis as well as facilitates complement activation. IgE fights infections with parasites and is also involved in autoimmune reactions and allergies. IgA antibodies are present in body secretions such as saliva and often defend against pathogens from inhaled and ingested pathogens first.
The next section will discuss the main functions of antibodies in more detail.
Antibodies are able to bind antigens on the surface of a pathogen also referred to as Pathogen associated molecular patterns PAMPs, effectively covering their surface and crosslinking them. This leads to precipitation of the pathogens, thus removing them from circulation and preventing further harm. This process is termed neutralization and occurs mostly in the mucosa of the mouth and upper airways and in the bloodstream.
Opsonization is the process by which toxins or pathogens are marked for destruction by phagocytosis. Numerous mediators of opsonization circulate in the human body, but the antibody response is the most important. Antibodies are able to essentially coat the pathogen’s surface and thus act as a bridge between the target cell and the phagocyte.
The complement system is part of the innate immune system and is separate from the adaptive immune system. It does not evolve with exposure to new pathogenic factors, but may be activated by the adaptive immune response. This activation is mediated by antibodies that are bound to their antigen. Complement proteins are subsequently cleaved into an active species by specific enzymes, leading to membrane attack, complex formation, phagocytosis and inflammation processes.
Over the past few years, the monoclonal antibodies and various recombinantly produced antibody fragments for medical and cell biology research have been consolidated into several antibody formats. Their characterization is based on structural features and/or on their antigen type. This section will give an overview over the four major antibody formats.
Antibodies that bind other antibodies’ idiotopes or variable regions are termed anti-idiotypic antibodies. They are very useful tools in assays to identify and research antibodies with specific antigen binding sites. Antibody testing is another application.
Bispecific antibodies are engineered to be highly specific binders to two distinct antigens or two epitopes of the same antigen. This format gave rise to promising therapeutic candidates with several constructs already in clinical trials and some already on the market. These variants of antibody and allied products are produced almost exclusively by recombinant methods and offer so much versatility to the types and formats that can be produced.
Read more on bispecific antibody production
Chimeric antibodies were developed to reduce the human immune response against therapeutic antibodies that were produced in other organisms, e.g., mouse antibodies. The basic approach is to keep the antigen-binding variable regions and replace the mouse-derived constant regions with their human counterparts. Developments in recombinant DNA technology has enabled the design of such chimeric antibodies.
Recombinant antibodies are considered to be the third generation of biotechnological antibodies, superseding traditional monoclonal antibodies and polyclonal antibodies. Unlike other methods of producing monoclonal antibodies (mAbs), recombinant antibody production is fully independent of laboratory animals. As an in vitro method, the production of recombinant antibodies is much more agile, faster and more reliable than traditional animal-based methods.
These significant advantages facilitated the development of numerous novel therapeutics, applications in diagnostics and tools for basic research.