Recombinant antibodies are mono-specific antibodies produced with recombinant DNA technology, which means they are generated in vitro using synthetic genes introduced into mammalian cell lines rather than through hybridoma cell culture or animal immunization.
Immunization of an animal or cultivation of hybridomas is not necessary. As recombinant antibodies are produced from a known DNA sequence, they have enhanced quality and reproducibility compared to polyclonal and traditional hybridoma-based monoclonal antibodies (Basu, Koli et al. 2019). Recombinant antibodies are used in medicine as well as in life science. They are therapeutic treatments for many diseases, including cancer or autoimmune disorders.
At evitria, we focus on delivering a recombinant antibody expression service for our international customers. Our experience and expertise in recombinant antibody production is based on over 120,000 CHO cell culture transfections and 20,000 antibodies purified. In this article, we would like to share 7 facts about recombinant antibodies. From the application in medicine and science to recombinant antibody production and the advantages of recombinant technology, this article will teach you all you need to know about recombinant antibodies.
Recombinant antibodies show mono-specific binding to a single epitope, just like a monoclonal antibody. Their production begins at the genomic level and is entirely in vitro. Using molecular biology techniques, synthetic genes coding for the antibody of interest are designed and introduced into cell lines.
Recombinant antibodies have been around since 1984, when Morisson SL. and Neuberger MS. cloned Ig genes from hybridomas to modify them in vitro. They expressed the first chimeric antibody, the first version of recombinant antibodies.
Subscribe to our Newsletter
Get all the latest updates, and learn about our advancements in antibody production.
Subscribe now
Advances in recombinant technology facilitated several breakthroughs, leading to the advent of recombinant antibody production on a large scale. Recombinant DNA technology allows the design and cloning of custom tailored genes into mammalian cells, which in turn produce antibodies in unprecedented quality and reproducibility. evitria is a leading service provider for recombinant antibody expression and custom recombinant antibodies – therefore, we rely on CHO cell expression.
Transient antibody expression in CHO (in vitro) even circumvents the questionable use of laboratory animals in contrast to in vivo antibody generation. If you make the Abs using a synthetic or human Ab library, then that eliminates the use of animals.
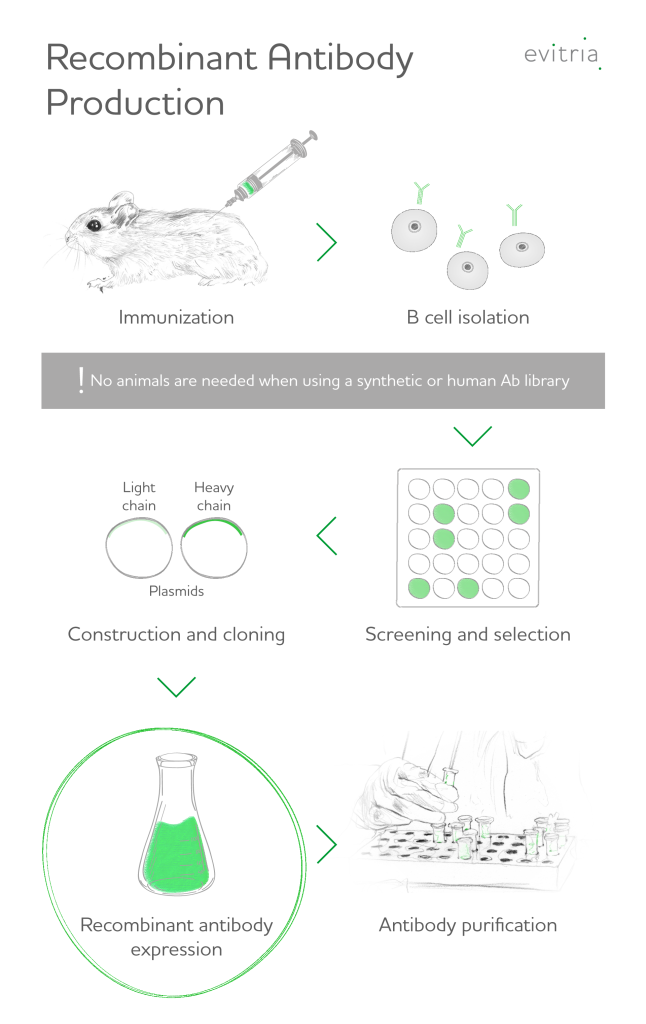
Recombinant antibody production commences with the isolation of promising genetic material (nucleic acids) coding for antibody candidates or the use of a library of genes with randomized antigen binding site sequences for antibody engineering.
Promising genes are introduced into expression vectors that display the associated antibodies on their surface (antibody phage display library technique). In the so-called panning technique, such a bacteriophage library is exposed to an immobilized antigen, the weak binders can be washed away from strong binders, which adhere to the antigen unless special reagents are used.
Repetition of this selection process with increasingly stringent conditions leaves only the strongest and most specific antibodies in the antibody library.
This in vitro process allows the manipulation of the genes to create new antibodies, reduce immunogenicity or even only select for antibody fragments (e.g., scfv or fab fragments).
Next, the most promising antibody genes are cloned into suitable cell lines that function as expression platforms, such as HEK or CHO cells: Since antibodies are rather complex proteins that undergo modifications after expression, higher cells (eukaryotic cells) are usually needed. Bacterial cells such as Escherichia coli would not yield the desired antibody products as they cannot perform post-translational modifications, such as the assembly of disulfide bridges, that are required for correct antibody production.
Most commonly, antibodies are produced with CHO cells (chinese hamster ovary cells) and HEK cells (human embryonic kidney). As a result of methodology research and ongoing mammalian cell culture optimizations, the yields of human antibodies in these expression systems could be improved to over 12 g / liter.1 CHO cells are the most widely used host for recombinant antibodies. About 70% of all recombinant proteins are produced in CHO cells stated by Feng Li et al.
Advantages of recombinant antibodies at a glance:
Find out more about the advantages:
Once a recombinant antibody is established and the optimal genetic sequence is known, the antibody can be produced at different scales with more predictable behaviour.
Further, the use of well-established expression platforms facilitates the upscaling of the necessary manufacturing processes, which are comparatively lean.
What is an antibody sequencing service?
Being an in vitro process entirely, recombinant antibody production is an agile technology that can switch between individual assignments on short notice. In contrast, the immunization of animals or production through hybridoma cell lines is more time consuming.
Additionally, in vitro processes are more economical and ethical since they do not require as many resources,produce as much waste, or use animals in the production process.
Once the amino acid sequence of the antibody is known, it can be edited and customized easily, allowing the properties of the recombinant antibody to changed for new applications. This is not possible when working with polyclonal or hybridoma-derived antibodies.
Since the underlying genetic material is easily optimized through the phage display method, recombinant antibodies can be designed to exhibit superior high affinity, sensitivity and specificity over traditional monoclonal antibodies.
Hybridoma cell lines are prone to spontaneous mutations, thus leading to potential consistency issues between batches. Polyclonal antibodies are known for lower reproducibility, since each batch is derived from individual animals.
Production of recombinant antibodies relies on entirely defined and well-controlled genetic sequences and thus yields highly consistent antibody products. This process leads to very good reproducibility and validation between batches.
Recombinant antibodies are used in numerous applications in medicine and life sciences, such as:
Recombinant antibodies offer remarkable versatility due to their ability to be customized and reformatted for specific applications. Let’s explore key insights into this exciting field and explore the customization methods we employ at evitria.
Chimerization involves combining the variable domains of an antibody (usually from a mouse, rat, or rabbit) with the constant domains from a different species (e.g., human). This results in a chimeric antibody that retains specificity while minimizing immunogenicity.
Chimeric antibodies bridge the gap between species, allowing us to harness the best features of each. For example, a humanized chimeric antibody can be used for therapeutic purposes without triggering adverse immune responses.
Traditional antibodies derived from animals can provoke immune reactions when administered to humans. By grafting the complementarity-determining regions (CDRs) of an antibody onto a human antibody framework, we create humanized recombinant antibodies. These retain specificity while minimizing immunogenicity, making them ideal for therapeutic use.
Isotype switching allows altering the isotype or subtype of an antibody. For instance:
Different isotypes have varying effector functions and stabilities. Isotype switching enables fine-tuning of an antibody’s properties.
In some situations, full-length antibodies may not be optimal. Recombinant expression platforms allow us to convert any antibody into fragments, followed by purification. Antibody fragments offer advantages:
If you need a unique format, we’re ready to guide you through the design choices.
As technology evolves, so do recombinant antibodies. From personalized medicine to targeted therapies, recombinant antibodies continue to shape the future of healthcare. Scientists, clinicians, and industry experts collaborate to unlock the full potential of these customizable molecules.
In summary, the customization and reformatting of recombinant antibodies empower us to tailor their properties for specific applications. Whether it’s humanization, chimerization, or creating antibody fragments, recombinant Abs offer a dynamic canvas for scientific innovation.
The scope of recombinant antibody applications is tremendous. The recent pandemic outbreak of SARS-CoV-2 virus had a big impact on everybody’s life. The strategies to fight the pandemic rely heavily on recombinant antibodies.
One example is the development of therapeutic antibodies that neutralize virus particles in infected patients. Recombinant antibodies that bind to surface proteins of SARS-CoV-2 are used in lateral flow test kits (“antigen tests”) to detect acute infections.
A recent publication of Canadian scientists in the Journal of Molecular Biology2 highlights the possibility for the synthesis of highly sensitive and specific reporter assays against SARS-CoV-2 virus particles using recombinant antibodies.
They created a conjugate reporter system consisting of two halves of a luminescence enzyme. Each half is attached to an antibody via a peptide linker. The antibodies were developed to have affinity to regions of viral surface proteins using recombinant and phage display technologies.
In the presence of the viral surface proteins, the antibodies bind to them, thus enabling the attached enzyme halves to reconstitute to a functional enzyme. The reporter system then emits light. This publication illuminates how rAbs can be rapidly developed into tools for important applications.
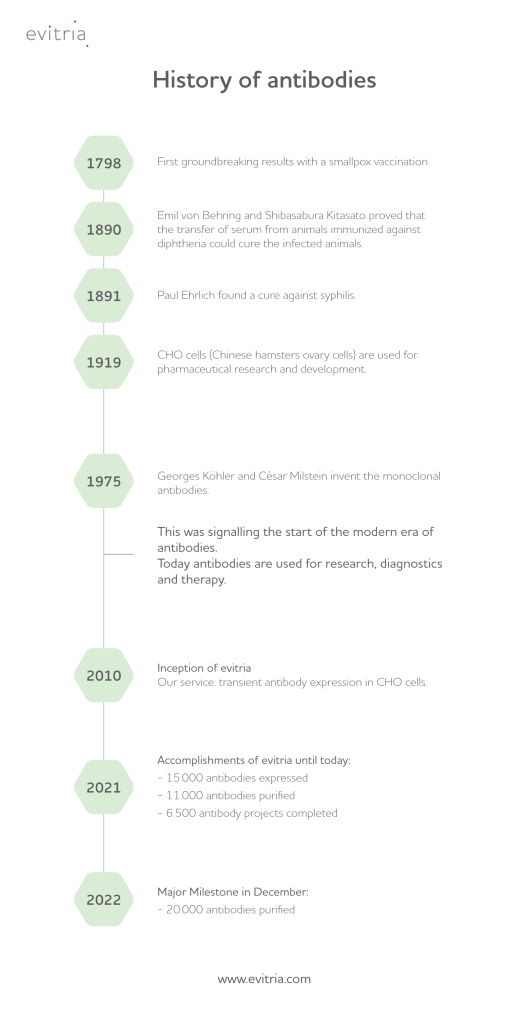
When it comes to the history of antibodies, the name Paul Ehrlich is inevitable, as it was him to discover a cure against syphilis and to coin the German term “Antikörper” in 1891. As a matter of fact, though, it was referenced already a year earlier by Emil von Behringand and Shibasabura Kitasato, as they found that animals infected with diphtheria can be cured by the transfer of serum from other, already immunized animals.
In 1975 Georges Köhler and César Milstein invent the monoclonal antibodies. This was the start of the modern era of antibodies. The first version of a recombinant antibody called “chimeric antibody” at that time was created by independently by Morrison SL. and Neuberger MS in 1984.
However, there is to know a lot more about the history of antibodies and the several milestones in this field that proved their immense relevance for life sciences and medicine, revolutionizing both areas significantly.
When it comes to reviewing which recombinant antibody is to selected, there are several factors to consider. They can be produced with different methods and tailor-made for specific applications. Nevertheless, there is no one-size-fits-all solution.This and more has to be considered when choosing which antibody is to be used.
At evitria, we use our CHO cells antibody production and expression platform for the generation of recombinant monoclonal antibodies. We can produce custom recombinant antibodies in large-scale within 5 weeks. Antibody production in CHO guarantees highest quality of recombinant antibodies and fast expression. You want to know more about our services? We would be pleased if you contact us.
Natural antibodies not only bind their respective antigen, they highlight it to the immune system as harmful and mark it for neutralization. This effect is called antibody dependent cellular cytotoxicity (ADCC) and is of high significance in the development of therapeutic antibodies in oncology.
Read more: Therapeutic afucosylated antibodies with enhanced ADCC
The underlying signalling process involves the recognition of a chain of carbohydrates on the antibodies. Research has shown that the nature of the carbohydrates influences the severity of the ADCC and that afucosylated antibodies (i.e., lacking fucose in the carbohydrate chain) have a particularly positive effect on ADCC.
Therefore, the ability to effect afucosylation of recombinant antibodies adds a whole new dimension to the scope of antibody technology. In addition to being able to develop highly specific antibodies to virtually any antigen through recombinant antibody technology, afucosylation allows the fine tuning of the strength of the immune system’s response to the antigen.
This fact opens the door to developing more potent and more tolerable therapeutic antibodies.
The production of high quality, reproducible material is critical for the development of antibody-based therapeutics. The evitria-Genovis workflow combines rapid, high quality, antibody production with high-throughput mass spectrometry for greater insight and control of key quality attributes.
Download the poster to learn more.

Antibodies (Abs) are proteins that belong to the class of immunoglobulins. They are naturally produced by certain types of blood cells of vertebrate animals and are part of the adaptive immune system. Humans produce several types of antibodies, with the so-called immunoglobulins G (IgG) and their various subtypes (such as IgG1) being the most common among them.
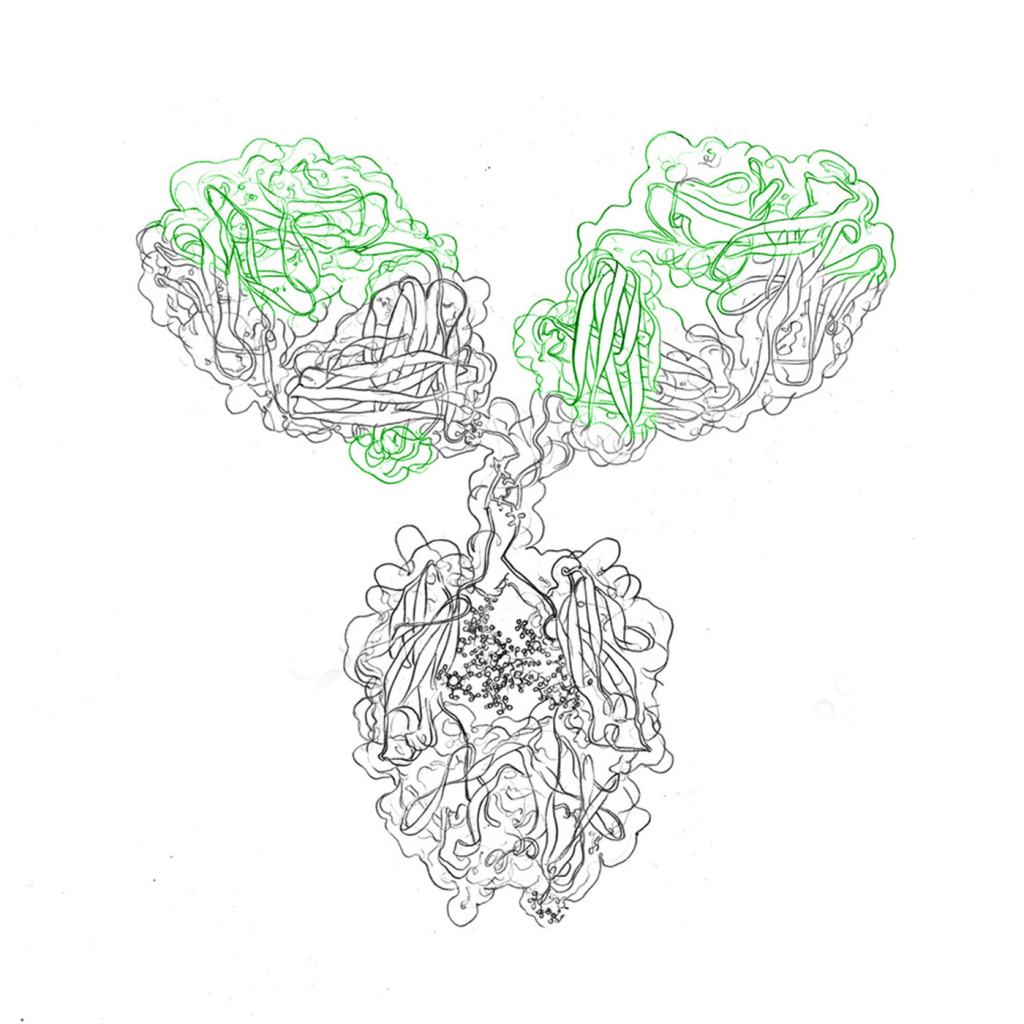
Antibodies are generally Y-shaped molecules consisting of heavy chains and light chains that bind to their target, or antigen, with a variable region. The antigen binding sites are located at the two tips of the “Y” and they mediate a very strong and specific binding interaction between antibody and antigen, similar to a receptor. The base of the “Y” is termed constant region (in contrast to the variable domain), although it’s sequence varies between species.
Antigens are usually parts of bacteria, microorganisms, or surface constituents of viruses. Antibodies bind to such pathogens, covering their surfaces, thus inactivating them and marking them as foreign to the immune system.
The high specificity of the antibody-antigen binding process led to the development of antibodies as valuable tools for diagnostic and therapeutic medicine and biological research. Nowadays, polyclonal, monoclonal and recombinant antibodies are available, and they come with their individual profile of pros and cons, depending on the specific application.
To trigger polyclonal antibody production, animals are injected with the antigen of interest. Further, repeat injections after initial animal immunization may be beneficial in order to increase the Ab titer in the blood.
Next, the polyclonal antibodies are harvested as solution in the serum by bleeding the animal and removing all blood cells. The polyclonal antibodies may be subjected to further purification by removing serum proteins. The choice of animal in antibody production depends on the desired amounts of Abs, their isotypes and immune response. Common animals for Ab production are rabbits, mice, horses, goats and llamas.
Key advantages of this process are the relatively low capital investment to obtain antibodies, but the use of animals poses ethical questions. Another disadvantage is the batch-to-batch variability, since they are obtained from different individual animals. Polyclonal antibodies are often used for immunoprecipitation, co-IP and ChIP applications.
Monoclonal antibodies (mAbs) are produced by single cells, unlike polyclonals which stem from a population of diverse B cells. The isolation of such a B cell for antibody production on large-scale is next to impossible and in addition, B cells have a relatively short expected life time.
A major breakthrough was the development of the hybridoma technology: B cells are harvested from immunized animals and then fused with myeloma cells. The resulting hybridoma cells carry the ability for antigen production of the B cell and the immortality of the myeloma cell. Hybridoma cells are then selected for specificity, and individual cells isolated.
Recombinant antibodies or recombinant monoclonal antibodies are antibodies that are produced by the means of recombinant antibody coding genes. They are monoclonal antibodies that are created in vitro, which means that hybridomas and animals are not needed. Find a full article on: What is a recombinant monoclonal antibody?
Recombinant antibodies are produced in a process called recombinant antibody expression, which is performed in vitro. By the means of genetic manipulation, new antibodies are created and then cloned into suitable cell lines. Read the full article about how are recombinant antibodies made.
One method for producing large numbers of identical antibodies is the hybridoma technology, which starts by injecting a mouse with an antigen to provoke an immune response. In recombinant antibody production, no animals are needed. When using a synthetic or human Ab library, there is no need for an immunization of an animal. No mice or hamsters are used in the recombinant antibody production! It does not require any animals in the production process as cell lines cultured in a lab are used as the base material.
While native antigens are produced in vivo, recombinant antigens are created artificially. A vector can be used to initiate their production, followed by their purification.
In biology, “recombinant” means that genetic material from different sources is combined. Read more: What is recombinant?
Recombinant DNA is produced by the means of different laboratory procedures in order to combine, split or rearrange DNA segments, resulting in a modification of the original DNA.
When an antibody is recombinant, this means that the antibody has been produced in vitro rather than by infecting living organisms. Instead, recombinant DNA is inserted into host cells by the means of a vector. This provokes the host cells to produce (recombinant) antibodies accordingly, which can then be harvested.
One prominent (and early) example of a recombinant is insulin; the genetic code for human insulin is inserted in bacteria, which are then “programmed” to produce insulin accordingly.