Monoclonal antibody technology revolutionized healthcare and the biopharma sector. Not only because of recombinant antibodies as their subclass, monoclonal antibodies (mAbs) have become indispensable as therapeutics for formerly incurable medical conditions, as assay components in diagnostics and as highly versatile tools in research.
This article will give you all information about monoclonal antibodies, highlight the dominant monoclonal antibody technologies and show some applications of mAbs that are timely in this SARS-COV-2 coronavirus pandemic as treatment options for COVID-19 patients.
Antibodies are Y-shaped glycoproteins that detect and bind strongly to their antigen (toxins, viruses, pathogenic bacteria). They are a crucial pillar of the human immune system which not only binds invaders, but activates immune responses, e.g. antibody-dependent cell-mediated cytotoxicity (ADCC) and recruits further key players such as phagocytes.
A monoclonal antibody is an antibody that stems from a single white blood cell clone. As such, monoclonals bind to a single epitope of a target antigen and are thus highly specific, whereas traditional polyclonal antibodies exhibit a broader binding spectrum to various epitopes of the same antigen, because they are produced by many individual B cells.
Subscribe to our Newsletter
Get all the latest updates, and learn about our advancements in antibody production.
Subscribe now
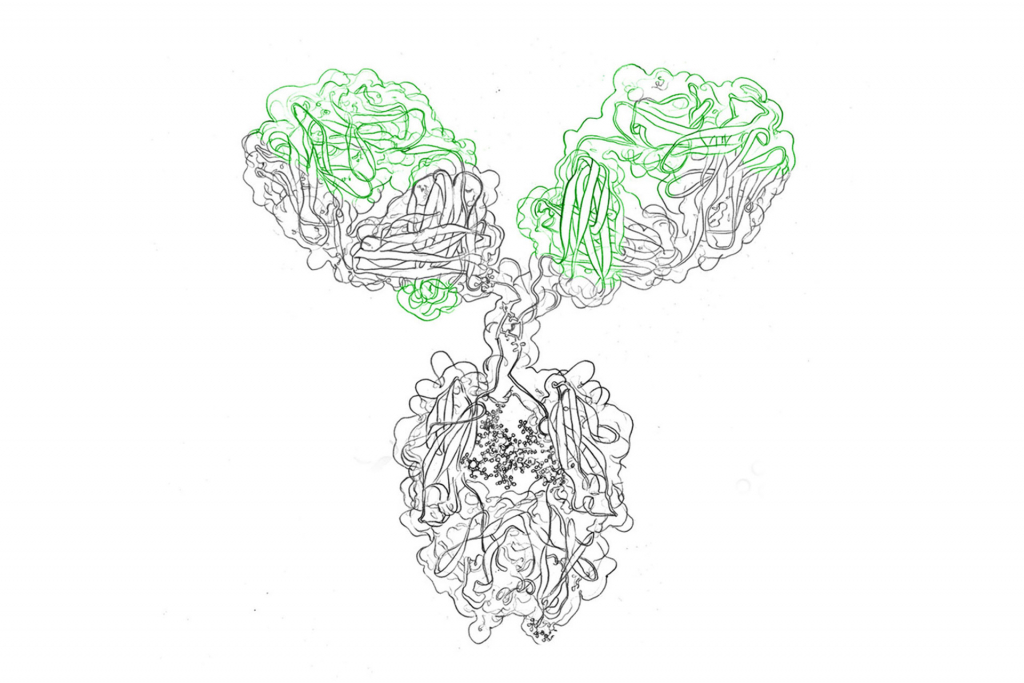
Advantages of monoclonal antibodies come with the possibility for engineering in the lab. While monoclonal antibodies only bind to one epitope, it is possible to enhance them by means of artificial engineering. By employing various techniques, labs can produce monoclonal antibodies that bind to a specific substance in order to detect or purify it.
These capabilities are just two of the benefits of monoclonal antibodies that add to their popularity in molecular biology, biochemistry and medicine.
The most important advantages are:
Under certain circumstances, the advantages that have been mentioned above can also be seen as disadvantages of monoclonal antibodies. Like two sides of a medal, certain strengths can turn into weaknesses, depending on requirements and point of view.
For one, the specificity of monoclonal antibodies naturally limits their scope of application. Due to their sensitivity, they are furthermore easily affected in terms of their functionality.
Other disadvantages of monoclonal antibodies may be that:
Being administered to patients as a therapeutic measure, monoclonal antibodies are able to cause different side effects. They can be respectively mild, like soreness or rashes, but also more severe. For instance, an overstimulation of the immunity system may result in the cytokine release syndrome or other strong immune responses.
Additionally, monoclonal antibodies used in cancer therapy may cause a tumor lysis syndrome, given their immense effectiveness.
Several technologies for monoclonal antibody production have been developed and the respective downstream processes optimized. Key breakthroughs were the development of antibody producing hybridoma cells, phage display technologies and Single B cell technologies. Now, we will take a closer look at each of them.
The basic concept of the hybridoma approach is the generation of identical immortal cells (a cell line) that produce one single antibody. This was first accomplished in 1975, when Milstein and Köhler fused antibody producing B cells of an immunized mouse with immortal myeloma cells to combine their desired properties in a single cell line: a hybridoma cell line that can be maintained indefinitely while harvesting their uniform, hence monoclonal antibodies.
The big disadvantages of this method are the involvement of animals during immunization, the risk of mutations in the antibody as the hybridoma ages, and the risk of additional variable domains being expressed by the cell line. In fact, a recent study screened 185 random hybridomas and found that over 30% of them produced additional heavy and light chains.1 Furthermore, hybridoma expression often requires a large amount of necessary labor, resulting in long lead times.
Phage display technology allowed the circumvention of several of hybridoma technologies’ disadvantages and speeding up the process of mAb generation. It was developed in the early 1990s by McCafferty and Winter and relies on molecular biology methods to genetically engineer bacteriophages to display antibodies on their surface.
This allows the binding of bacteriophages with desired antibodies onto immobilized antigens and thus enriching them during multiple washing steps. The high affinity binders are then used to infect bacteria and collect their DNA for sequencing. This genetic data allows the design of genes to be used in antibody production in cell cultures.
The overall process is much faster than hybridoma cell generation and doesn’t need any laboratory animals.
Single B cell technologies are another milestone in mAb development. The technique involves the screening and sorting of ex vivo cultures of single B cells from blood samples of immunized animals or humans to select for promising antibody candidates.
Genetic information is then extracted from the candidate cells’ mRNA after reverse transcription into cDNA, amplification and subsequent sequencing and cloning into expression vectors for mAb production.
This method bypasses the need to create a hybridoma cell line, and so shortens the development timeline by several weeks. Furthermore, by removing the hybridoma stage, the efficiency of the discovery process is increased – no candidate antibodies are “lost” due to failed hybridoma fusion or mutation in the hybridoma formation process. This translates into a higher number of candidates being discovered when using a single B cell approach – especially for difficult to target proteins (GPCRs, low-expressed membrane transporters) where a 10-fold increase in leads can be obtained.
Further readings:
Monoclonal antibody production – how are monoclonal antibodies produced
Who produces monoclonal antibodies?
Critical steps in monoclonal antibody development
evitria AG is a leading service provider for recombinant monoclonal antibody expression in CHO cells. Recombinant antibodies are generated in vitro without the use of animals in the lab. Recombinant technology offers best quality, consistency and batch-to-batch reproducibility making the process highly scalable. evitria offers high quality recombinant antibodies from sequence to antibody within 5 weeks.
MAbs are used in a variety of fields all over life sciences and medicine. Predominantly, they play out their advantages in diagnostics and when used for therapeutic purposes.
The diagnostic applications of monoclonal antibodies include
Additionally, the therapeutic application includes their use in cancer therapy and the treatment of autoimmune diseases.
Further readings:
Fields of application of monoclonal antibodies
Therapeutic monoclonal antibodies – powerful biological drugs
Modern monoclonal antibody technology facilitated the quick development of monoclonal antibody therapies with affinities to the spike proteins to treat COVID-19.
Several monoclonal antibody treatments (mAb treatments) with promising data from clinical trials received emergency use authorizations (EUA) by the US Food And Drug Administration (FDA): bamlanivimab plus etesevimab (intravenous administration), casirivimab plus imdevimab (REGEN-COV, subcutaneous administration) and sotrovimab (superior effectivity against the omicron variant) for the treatment of moderate COVID-19 in outpatient treatment settings with high risk of progression to severe disease (e.g. pre-existing lung disease, high blood pressure).
Tixagecimab plus cilgavimab (evusheld) received EUA status for pre-exposure prophylaxis in patients who are expected to have severe side effects to COVID-19 vaccines (e.g. allergic reactions) and immunocompromised patients who are expected to have an inadequate response to vaccination and are at risk for severe COVID-19 infections.2
The fast development and upscale of these treatments of COVID-19 are impressive feats of biopharma innovators, and having them in the arsenal of health care providers is a boon to public health.
Further readings:
What is a monoclonal antibody infusion?
How effective are monoclonal antibodies in terms of COVID-19?
Some people object to drug trials involving humans as they see an ethical issue in the potential risks, even though all subjects taking part in trials do so voluntarily, and they often get paid for their participation.
Besides general questions of morals and religion, discussions about ethical issues in the pharmaceutical industry often revolve around animal rights.Naturally, this is no different when discussing monoclonal antibodies whose origins can be traced to CHO cells or mouse cells. The use of animals – either as testing subjects or for production purposes – can be deemed unethical by some people.
However, animal cell culture technology has advanced significantly over the last few decades. There are strict guidelines in place to regulate the use of animals where it is still necessary.3
Additionally, if various scientific publications/articles4 are to be believed, the use of animals for the expression of antigen-specific antibodies is on the decline. This is owed to the fact that recombinant antibodies, derived from synthetic genes, are on the rise.
But what exactly are recombinant antibodies? Recombinant antibodies are monoclonal antibodies that are generated in vitro using synthetic genes.
While monoclonal antibodies are derived from CHO cells and other mammalian cell culture, no hamster, mouse or other animal is used, let alone harmed, in the process of recombinant antibody expression. Apart from being ethically sourced, recombinant antibodies offer versatility as another major advantage. The most commonly used recombinant antibodies are scFv, Fab fragments and bispecific antibodies, the latter of which are more varied than monoclonal antibodies as they increase the therapeutic targets of one monoclonal antibody to two epitopes.
What is a recombinant monoclonal antibody?
Monoclonal antibodies are made by cloning a white blood cell, which is always unique. All subsequent antibodies derived this way trace back to this unique parent cell, while polyclonal antibodies are usually derived from several different plasma cell lineages.
Read more:
As described by the FDA, allocated in the U.S. Department of Health and Human Services, monoclonal antibodies may be effective in the treatment of Covid-19 by inhibiting the virus from using body cells for reproduction.
There are several monoclonal antibody products that have been allowed to be used in the treatment of Covid-19, including casirivimab/imdevimab, Bamlanivimab/etesevimab, and sotrovimab.
A monoclonal antibody example is rituximab: The product is used in cancer therapy, as these antibodies are able to bind to certain proteins on specific cancer cells. The immune system is then supposed to recognize these cells, and consequently to combat them.
Monoclonal antibodies are used in several fields of life sciences and medicine, Typically, they are chosen for diagnostic or therapeutic applications.
Monoclonal antibodies are generally considered to be safe and effective pharmaceutical products. Nevertheless, there are risks to be considered before administration, e.g. unwanted immune reactions, as well as adverse effects. Yet, monoclonal antibodies usually cause less severe side effects than conventional chemotherapy products.
According to information of National Institute of Health (NIH), there are dedicated monoclonal antibodies available for COVID-19, also for the current omicron variant. In terms of prevention, Tixagevimab plus cilgavimab (Evusheld) may be considered for administration to certain groups of people.
On the other hand, clinical studies highlight an effectiveness of Bebtelovimab in the treatment of an infection with SARS-CoV-2. Usually, these products are administered in infusion centers; the CDC provides further information on who may be eligible for mAb application in association with COVID-19.
As for today, more than a hundred monoclonal antibody products are approved by FDA.
One can distinguish three different types of monoclonal antibodies after long-term immunizing rats with mouse nerve growth factor:
Source: Cattaneo, A. et al.: “Three distinct types of monoclonal antibodies after long-term immunization of rats with mouse nerve growth factor”, Journal of Neurochemistry