Afucosylated Antibody Expression
Learn more about our afucosylated antibody expression service
Afucosylated antibodies are recombinant monoclonal antibodies with increased antibody-dependent cellular cytotoxicity. They play a vital role in immunotherapy and cancer treatment, as they can more effectively target and eliminate cancer cells while minimizing harm to healthy tissues. Their precision and potency make them valuable tools in the fight against various diseases.
Afucosylated monoclonal antibodies represent a novel class of therapeutic antibodies engineered to have reduced fucose content in their glycan structures. Fucose is a sugar molecule found in the Fc region of antibodies, and its removal results in afucosylated antibodies. This modification enhances their binding affinity to Fc gamma receptors on immune cells, such as natural killer cells and macrophages, amplifying their ability to trigger immune responses. Japanese scientists showed that the lack of fucose and not the presence of galactose is crucial for the ADCC effect.1
In general, antibodies are Y-shaped proteins, also known as immunoglobulin G (human IgG), and are produced by immune cells termed B cells in the human body. As they are able to identify and neutralize foreign objects – in other words viruses – they play a major contributing role in keeping our body and immune system healthy.
Their ability to target almost any cell surface or secreted molecule with remarkable binding affinity and safety contributes to their popularity, and there is a tremendous amount of research being conducted around immunology and antibodies.
Subscribe to our Newsletter
Get all the latest updates, and learn about our advancements in antibody production.
Subscribe now
Today, various types of antibodies can be reproduced and cultivated in labs to then be utilized in a number of medical diagnostic assays and pharmaceutical applications. In fact, they represent the fastest growing group of biotherapeutics both in terms of clinical trials and in global sales revenue.
Since afucosylated & recombinant antibodies have been approved and commercially used for the first time in the late 1980s, artificially cultivated antibodies have seen a steady rise in terms of utilization. Currently, there are several hundred products in development, many of which are targeting multiple indications. This whole process is called “Glycoengineering of Antibodies for Modulating Functions” and involves the creation of glycoproteins, combining certain enzymes with carbohydrates.
So what’s the impact of glycosylation on monoclonal antibodies (mAbs)? Do recombinant and afucosylated antibodies live up to the hype surrounding them, and what are (potential) fields of application?
Let’s start with recombinant antibodies (rAbs): Those are monoclonal antibodies that are generated in vitro using synthetic genes and biotechnology. In the past decade alone, several antibodies have been developed for a number of therapeutic applications and several known formats of recombinant antibodies are produced, with each of the formats having a slightly different potential in applications and fields of research (e.g. inhibitory effects on cellular pathways, fluorescence assays, flow cytometry, exp. profiling of proteins with chromatography – mass spectrometry). The most commonly used recombinant antibodies are scFv, Fab fragments and bispecific antibodies. An advantage of recombinant antibodies (rAbs) is their high affinity compared to low-affinity bodies as they bind strongly to the antigen and maintain better under difficult conditions.
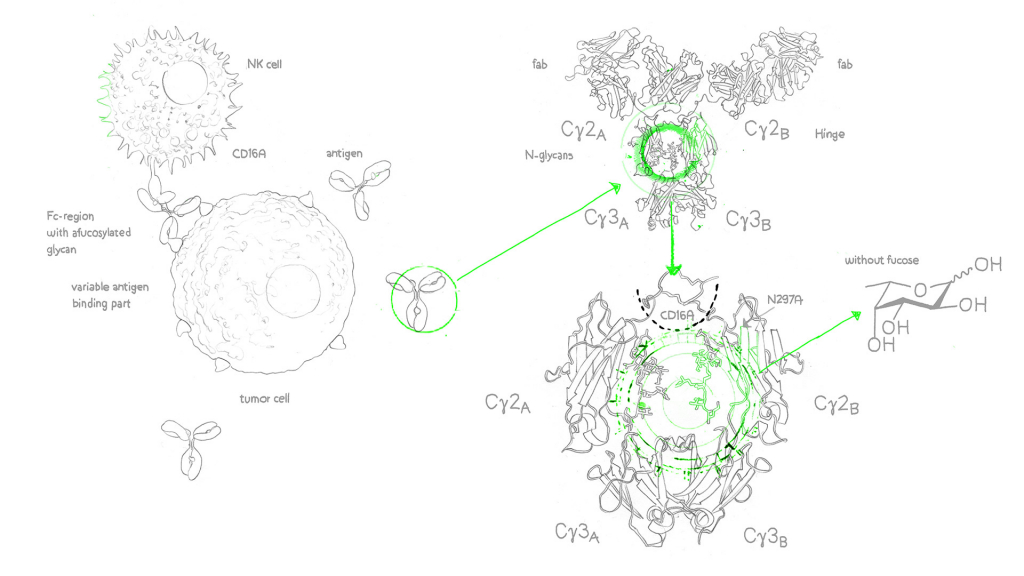
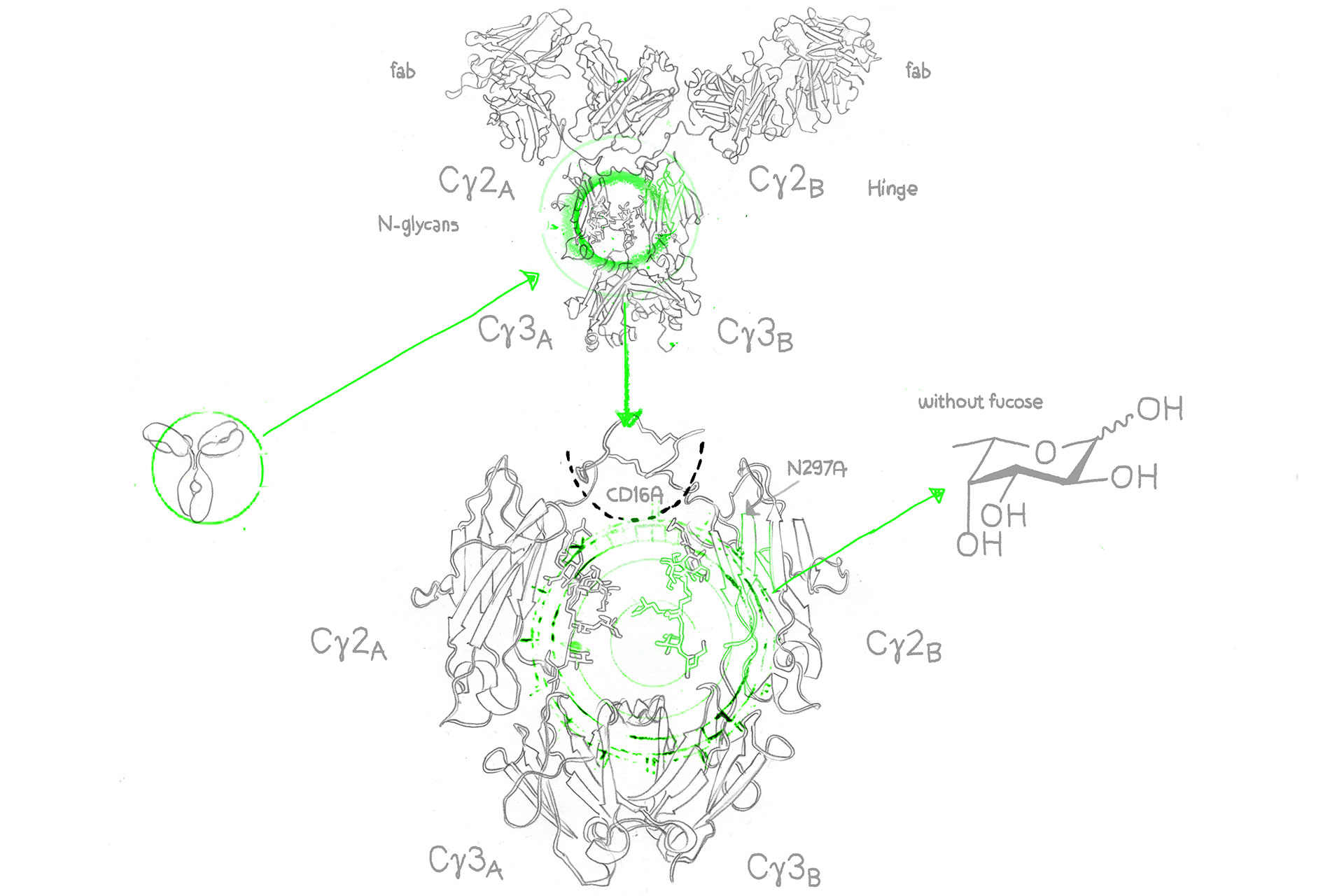
Afucosylated antibodies on the other hand are engineered monoclonal antibodies (mAbs) with the n-glycan (oligosaccharide and sialylation) residues in the IgG Fc region missing core fucose sugar units, a fact that contributes to the increase of the antibody-dependent cellular cytotoxicity (ADCC).2
Enhanced ADCC activity is important to push the efficacy of cancer antibodies, however, as a result of nonspecific IgG1 inhibition of FcγR/fc gamma receptor (fcγ of the fcγIIIa isotype) binding on lymphocytic effector cells such as natural killer cells (NK cells)3, a number of already approved cancer antibodies modulate ADCC less than could be desired. And this is where the afucosylated monoclonal antibody comes in: It overcomes this problem through enhancement of FcγIIIa binding – the results are (antibody-dependent cellular cytotoxity) ADCC enhanced antibodies.4
Recombinant antibodies are not only easily and reliably reproduced but offering a number of additional advantages, which is why they are increasingly popular in both medical and research applications. More currently speaking, they are being cultivated on the basis of genetically modified E. coli, or preferably CHO (Chinese Hamster Ovary) cells – due to native glycoforms – to be applied in various therapeutics and research studies.
Pharmacological characterization of afucosylated antibodies for therapeutic use show more potency in vitro and in vivo than their fucosylated counterparts – such as recombinant antibodies – resulting in the likelihood of making them more powerful and efficient in designing next-generation therapeutic antibodies.5 Thanks to their low fucose content, afucosylated antibodies exhibit increased killing kinetics against tumorigenic and infected cells.
Afucosylated antibodies find diverse applications across the field of biomedicine:
Learn more about our afucosylated antibody expression service
At evitria, recombinant antibody production is based on the clients’ individual needs and requirements. The antibodies are generated in vitro using synthetic genes derived from CHO cells (Chinese Hamster Ovary).
Over the years those have remained a popular choice with many biopharmaceutical manufacturers as they are ideal for large-scale cultures while at the same time offering high recombinant protein yields and specific productivity: CHO cells can produce recombinant protein on a scale of 3-10 grams per liter of CHO cells culture, superior to HEK293 cell in PBS media.
Afucosylated antibodies or ADCC antibodies are merely modified recombinant antibodies with the benefit of exhibiting an artificially decreased fucose content, which leads to the effector function of ADCC and results in an elevated killing activity against tumorigenic and infected cells. At evitria, antibody expression takes place using the same CHO cells and DNA as native antibodies.
Thanks to their efficient and reliable ability to target almost any cell surface or secreted molecule, genetically modified as well as native antibodies offer unparalleled opportunities for the pharmaceutical and life-science industries. This furore is causing a high demand that can be easily met, thanks to easy cultivability and ever improving cultivation systems and processes.
Speaking of which: The state-of-the art GlymaxX® platform enables evitria to transiently express both native and afucosylated variants using the same CHO cell line, the same DNA, and without any amino acid sequence modifications. So while alternative afucosylation approaches rely on Fc mutations with the risk of immunogenicity and cytokine release syndrome or on patented FUT8 knockout methods, evitria’s use of GlymaxX® produces the same yields and stability as native antibodies – but ruling out the danger of potential immune response.
Recommended readings from Desmond Schofield:
Afucosylation sipmly explained
Antibody-Dependent Cellular Cytotoxicity (ADCC) – definition, mechanism & application
Afucosylated Antibody Expression Service
Glycoengineering: pushing forward biotechnology
Afucosylation is a biochemical modification that involves altering the glycan structures of molecules, particularly glycoproteins, by removing fucose sugar residues. Fucose is a monosaccharide (simple sugar) that can be found in various glycans attached to proteins.
Glycoengineering is the controlled modification of sugar molecules (glycans) on biomolecules like proteins and lipids. It allows scientists to customize and enhance the functions of these biomolecules for various medical and biotechnological applications, including improving the effectiveness of therapeutic antibodies like afucosylated antibodies in cancer treatment.
Core fucose is a sugar molecule on antibodies. Reducing core fucose in afucosylated antibodies enhances their effectiveness in recruiting immune cells.
FcγRIII, or Fc Gamma Receptor III, refers to a family of immune receptors found on the surface of immune cells. These receptors play a crucial role in binding to the Fc region of antibodies, initiating immune responses. FcγRIII includes various variants, with FcγRIIIa and FcγRIIIb being notable members.
FcγRIIIa, or Fc Gamma Receptor IIIa, is a specific variant within the FcγRIII family of receptors. It is prominently found on immune cells like natural killer (NK) cells, macrophages, and neutrophils. FcγRIIIa plays a pivotal role in enhancing immune responses by binding to the Fc region of antibodies, facilitating processes like antibody-dependent cellular cytotoxicity (ADCC) and phagocytosis.
Fucosyltransferase is an enzyme responsible for adding fucose sugar molecules to glycoproteins and glycolipids. This modification plays crucial roles in cell signaling, immune responses, and various cellular functions by altering the structure and function of these molecules.