Recombinant monoclonal antibody production
We are a leading service provider for recombinant antibody production. Get in contact!
Antibody production in bacteria offers numerous benefits, but also unique challenges. Bacteria, such as E. coli and Bacillus subtilis, provide rapid growth, genetic manipulability, and cost-effectiveness. Harnessing these microbial systems, researchers have successfully engineered bacteria to produce high yields of functional antibodies.
Understanding the structure and function of antibodies is crucial. Composed of heavy and light chains, antibodies possess variable regions that bind to specific antigens, enabling targeted recognition. Bacterial expression systems, utilizing vectors like plasmids or phages, facilitate the production of recombinant antibodies with desired specificity and affinity.
In this article, we will explore the process of antibody production in bacteria, including genetic modification, optimization of expression levels, and antibody engineering. Additionally, we will discuss purification and characterization techniques, along with the diverse applications of antibodies produced in bacteria. By leveraging the power of bacteria, antibody production has reached new heights in biotechnology and promises exciting possibilities for the future.
Antibodies, also known as immunoglobulins, are essential components of the immune system and play a pivotal role in recognizing and neutralizing foreign substances, known as antigens. Understanding the structural characteristics of antibodies is fundamental to comprehend their diverse applications in research, diagnostics, and therapeutics.
Subscribe to our Newsletter
Get all the latest updates, and learn about our advancements in antibody production.
Subscribe now
Full-length antibodies consist of two identical heavy chains and two identical light chains. The heavy chains contain constant and variable regions, while the light chains contribute to the antigen-binding site. These antibodies are commonly produced through hybridoma technology, a time-consuming process involving the fusion of a specific B cell with a myeloma cell to generate monoclonal antibodies.
Antibody fragments offer advantages over full-length antibodies, such as improved tissue penetration and reduced immunogenicity. Recombinant antibody technologies have enabled the production of antibody fragments, including single-chain variable fragments (scFv antibodies) and single-chain antibodies (scAbs). These fragments retain the antigen-binding capabilities of full-length antibodies while exhibiting enhanced biochemical properties.
Antibodies possess specific binding sites, known as paratopes, which interact with antigens. The variable regions of the heavy and light chains contribute to the formation of the antigen-binding site. Through genetic engineering techniques, such as codon optimization and recombinant DNA technology, researchers can modify and tailor the binding site to enhance affinity and specificity.
Monoclonal antibody production involves the generation of identical antibodies derived from a single B cell clone. Hybridoma technology, combined with biochemical assays like enzyme-linked immunosorbent assay (ELISA), facilitates the screening and selection of high-affinity monoclonal antibodies. These antibodies have revolutionized various fields, including diagnostics, therapeutics, and research.
Genetic engineering allows the creation of chimeric and recombinant antibodies by combining different antibody components. Chimeric antibodies possess human constant regions fused with variable regions from other species. Recombinant antibodies are entirely human, generated using phage display or other recombinant technologies. These approaches provide enhanced compatibility and reduced immunogenicity for therapeutic applications.
Antibody production in bacteria has emerged as a powerful and versatile approach in the field of biotechnology. By harnessing the natural protein expression systems of bacteria, researchers can efficiently produce a wide range of antibodies and recombinant proteins. Bacterial expression systems, particularly those utilizing the popular E. coli strain, have become a frequent choice for antibody production due to their simplicity, scalability, and cost-effectiveness.
In vitro production of antibodies in bacteria involves the expression of antibody genes within the bacterial host. This process typically begins with the introduction of the antibody gene into the bacterial genome or a plasmid vector. E. coli strains, such as BL21(DE3), have proven to be highly efficient hosts for the expression of recombinant proteins, including therapeutic antibodies.
E. coli-based expression systems have been extensively optimized for antibody production, allowing for the efficient expression and secretion of antibodies into the culture medium. This simplifies downstream purification processes, reducing the time and effort required for antibody isolation.
The production of therapeutic antibodies in bacteria has revolutionized the biopharmaceutical industry. Antibodies with therapeutic potential can be engineered and produced at large scales in bacterial hosts, offering a cost-effective means of providing life-saving treatments.
However, the expression of antibody genes in bacteria requires careful consideration of various factors. These include the choice of promoter, host strain, and cultivation conditions to ensure optimal expression levels. Furthermore, downstream processing steps, such as protein extraction and purification, are crucial to obtain pure and functional antibodies.
Bacterial expression systems have played a pivotal role in revolutionizing the production of recombinant proteins, including antibodies, in the field of biotechnology. Among the various bacterial hosts, Escherichia coli (E. coli) stands out as one of the most widely used and well-characterized organism for this purpose.
The cloning of antibody genes into bacterial expression vectors enables the production of recombinant proteins within the bacterial host. Once inside the host, the genetic information is transcribed and translated into proteins. In the case of E. coli, protein expression can occur either in the cytoplasm or through secretion into the extracellular space.
The cytoplasmic expression of proteins in E. coli is a straightforward and efficient process. It involves the insertion of the antibody gene into a plasmid vector under the control of a strong promoter. This enables high-level synthesis of the desired protein within the bacterial cytoplasm.
Alternatively, secretion systems can be utilized to direct the expression and secretion of recombinant proteins, including antibodies, into the extracellular space. E. coli has several well-characterized secretion systems, which facilitate the efficient export of proteins.
Besides E. coli, other bacterial hosts, such as Bacillus subtilis, have also been employed for antibody production. Bacillus-based systems offer advantages such as robust protein synthesis, secretion capabilities, and the ability to perform large-scale fermentations.
Phage-based systems provide an additional approach for antibody expression. By employing bacteriophages, specifically engineered to display antibody fragments, researchers can create libraries of monoclonal antibodies (mAbs) for screening and selection. This technology has greatly facilitated the discovery and development of therapeutic mAbs.
Antibody engineering and optimization play a crucial role in enhancing the production, functionality, and stability of antibodies. By manipulating various aspects of the antibody structure and expression process (not only based on bacteria), researchers can tailor antibodies to meet specific requirements and improve their efficacy.
Antibody purification and antibody characterization are further critical steps in the production process to obtain pure and functional antibody preparations. These processes involve separating the antibody of interest from other cellular components and assessing its quality, stability, and biological activity.
Antibodies produced in bacteria have revolutionized research, diagnostics, therapeutics, and industrial applications. They serve as valuable tools for protein detection, localization, and quantification in research, aiding in understanding cellular processes and protein interactions. In diagnostics, bacterial-produced antibodies are used in assays to detect specific antigens or markers, facilitating disease diagnosis and monitoring.
Therapeutically, antibodies produced in bacteria, particularly monoclonal antibodies (mAbs), have shown promise in treating cancer, autoimmune disorders, and infectious diseases. They also play a vital role in antibody-drug conjugates (ADCs), delivering targeted therapy with reduced side effects.
The production of antibodies in bacteria presents challenges and offers exciting future prospects. Overcoming these challenges and exploring new avenues are crucial for optimizing antibody production and expanding their applications.
Efforts are underway to improve transcription and expression efficiency for IgG antibodies and other formats. Achieving native-like post-translational modifications and enhancing effector function and stability are areas of active research. Antibody engineering, including novel formats and modifications, holds promise for targeted therapies. Quality control measures and integration of upstream and downstream processes are essential for ensuring high-quality antibody preparations.
Personalized medicine and tailored antibody therapeutics based on individual patient profiles represent an exciting frontier. And next-generation expression systems, such as yeast, mammalian cells, and plant-based platforms, offer advantages in post-translational modifications and scalability.
In addition to bacterial expression systems, alternative platforms offer valuable alternatives for producing antibodies and recombinant proteins. These systems, such as mammalian cells, Saccharomyces cerevisiae, Chinese hamster ovary (CHO) cells, and other microbial hosts, present unique advantages for specific applications.
We are a leading service provider for recombinant antibody production. Get in contact!
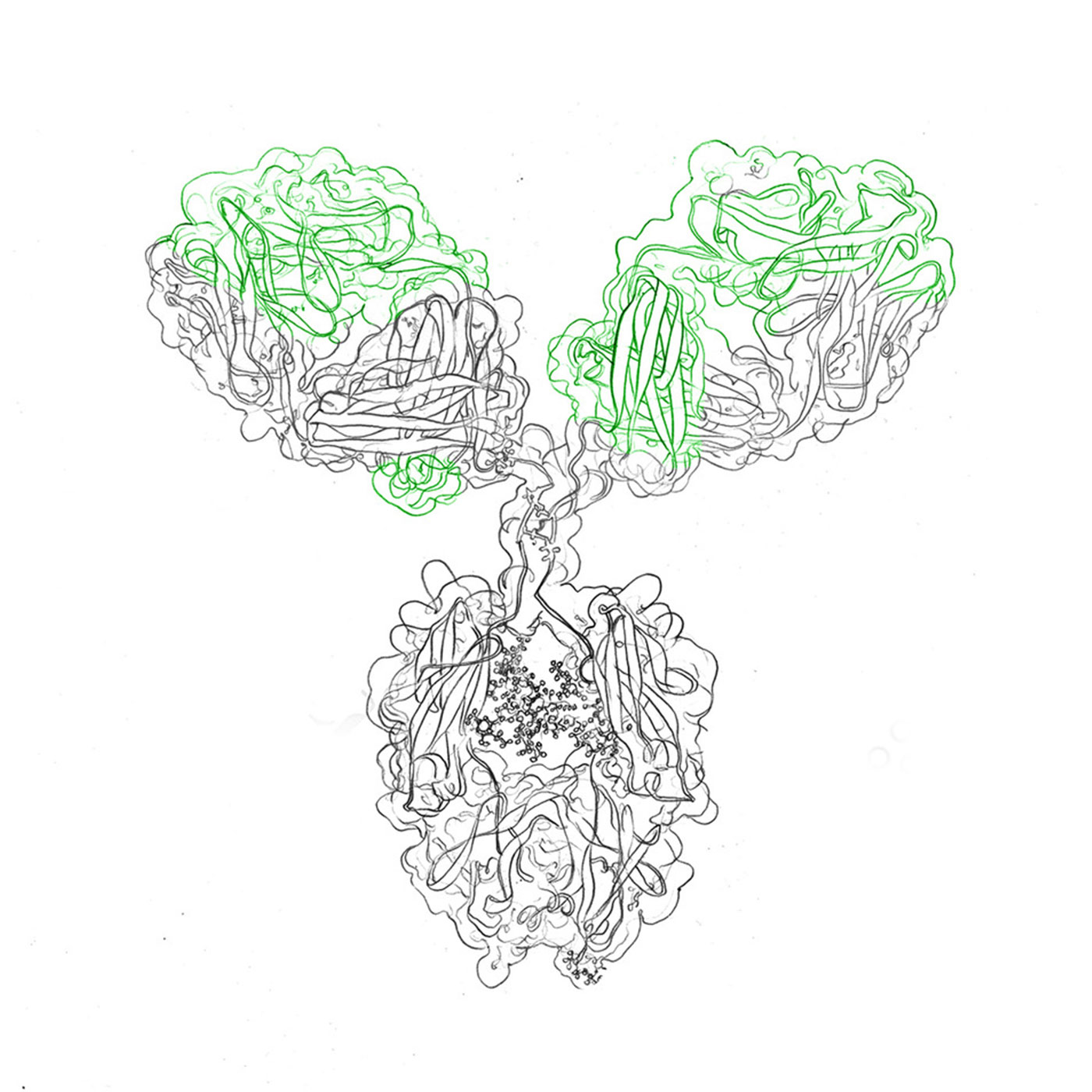
Other microbial hosts, such as various bacterial strains and fungal systems, also offer potential for recombinant protein expression. These systems are often characterized by rapid growth, simplicity, and cost-effectiveness, making them attractive for certain applications. Furthermore, the development of new cell lines and optimization strategies continues to expand the capabilities of microbial expression systems.
Saccharomyces cerevisiae, a yeast expression system, has gained attention for its capacity to produce recombinant proteins efficiently. This system offers benefits such as low-cost media requirements, ease of genetic manipulation, and compatibility with high-density fermentation processes. Yeast expression systems are well-suited for producing antibody fragments and other proteins with specific applications.
Mammalian cells are particularly advantageous for the production of complex proteins, including antibodies. Mammalian cell cultures possess the necessary cellular machinery for proper protein folding, post-translational modifications, and assembly into functional antibody structures. Mammalian expression systems are often employed for large-scale production of therapeutic antibodies due to their ability to generate high-quality, biologically active molecules.
Chinese hamster ovary cells (CHO cells) are widely used in the biopharmaceutical industry for large-scale production of therapeutic proteins, including antibodies. This is why we at evitria incorporate CHO cells in our recombinant antibody production services: In antibody production, CHO cells provide high protein expression levels, proper folding, and the ability to perform complex post-translational modifications.
These attributes make CHO cells our preferred choice for recombinant antibody production, providing partners worldwide with custom-made antibody products.